Unifying serological testing for cold agglutinins
- PMID: 40930510
- PMCID: PMC12422832
- DOI: 10.1111/vox.70069
Unifying serological testing for cold agglutinins
Abstract
Background and objectives: Cold agglutinins (CAs) are immunoglobulin M autoantibodies that optimally bind to red blood cells at low temperatures. The clinical significance of CAs is usually characterized by the CA titre and thermal amplitude. However, there is no consensus on the optimal testing strategy. In this study, we sought to unify CA testing.
Materials and methods: A survey on CA evaluation was conducted in 17 Dutch transfusion laboratories. Additionally, samples from 16 patients with CAs were used to evaluate (pre)analytical variations in test outcomes.
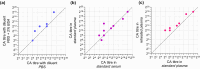
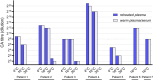
Results: The survey on CA evaluation revealed heterogeneity in the indications for CA workup and in (pre)analytical conditions. Titration results between EDTA plasma and serum did not differ. However, titres were higher when 2% bovine serum albumin (BSA) was present in the titration medium. Routinely collected EDTA-blood kept at 37°C for 10 min before plasma isolation (reheated) showed equivalent results to plasma from blood kept at 37°C from collection through separation.
Conclusion: This study showed that different (pre)analytical conditions influence test outcomes in the characterization of CAs. Importantly, CA testing can effectively be performed with reheated blood samples, which will facilitate a more practical workflow for laboratories. International standardization of CA testing for diagnostic use is essential.
Keywords: autoimmune haemolytic anaemia; cold agglutinin disease; cold agglutinins.
© 2025 The Author(s). Vox Sanguinis published by John Wiley & Sons Ltd on behalf of International Society of Blood Transfusion.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures
References
-
- Petz LD, Garratty G. Acquired immune hemolytic anaemia. 2nd ed. Philadelphia: Churchill‐Livingstone; 2004.
-
- Garratty G, Petz LD, Hoops JK. The correlation of cold agglutinin titrations in saline and albumin with haemolytic anaemia. Br J Haematol. 1977;35:587–595. - PubMed
-
- Berentsen S. How I treat cold agglutinin disease. Blood. 2021;137:1295–1303. - PubMed
-
- Jalink M, Yan MTS, Cohn CS, Eichbaum QG, Fung MK, Lu W, et al. Systematic review for the serological testing for cold agglutinins: the BEST collaborative study. Transfusion. 2024;64:1331–1349. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

