Modulating the PPARγ pathway upregulates NECTIN4 and enhances chimeric antigen receptor (CAR) T cell therapy in bladder cancer
- PMID: 40931013
- PMCID: PMC12423289
- DOI: 10.1038/s41467-025-62710-0
Modulating the PPARγ pathway upregulates NECTIN4 and enhances chimeric antigen receptor (CAR) T cell therapy in bladder cancer
Abstract
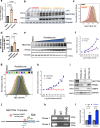
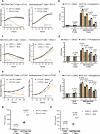
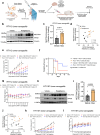
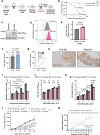
With the approval of the antibody-drug conjugate enfortumab vedotin (EV), NECTIN4 has emerged as a bona fide therapeutic target in urothelial carcinoma (UC). Here, we report the development of a NECTIN4-directed chimeric antigen receptor (CAR) T cell, which exhibits reactivity across cells expressing a range of endogenous NECTIN4, with enhanced activity in high expressors. We demonstrate that the PPARγ pathway, critical for luminal differentiation, transcriptionally controls NECTIN4, and that the PPARγ agonist rosiglitazone primes and augments NECTIN4 expression, thereby increasing sensitivity to NECTIN4-CAR T cell-mediated killing. NECTIN4-CAR T cells have potent anti-tumor activity even against EV resistant cells, which largely retain NECTIN4 expression, including in a post-EV biopsy cohort. Our results elucidate a therapeutically actionable mechanism that UC cells use to control NECTIN4 expression and suggest therapeutic approaches that leverage PPARγ agonists for rational combinations with NECTIN4-targeting agents in UC, as well as future potential treatment options for EV-refractory patients.
© 2025. The Author(s).
Conflict of interest statement
Competing interests: D. Solit has consulted/received honoraria from Rain Pharmaceuticals, Pfizer, Fog Pharma, PaigeAI, BridgeBio, Scorpion Therapeutics, FORE Therapeutics, Function Oncology, Pyramid, Elsie Biotechnologies, Inc, and Meliora Therapeutics, Inc, all of which are outside the submitted work. H. Al Ahmadie has consulted for AstraZeneca and Paige.AI, all of which are outside the submitted work. J. Rosenberg served in a consulting or advisory role for Aktis, Astellas, AstraZeneca, Bayer, Boehringer Ingelheim, Bristol-Myers Squibb, Century Therapeutics, EMD Serono, Gilead, Century Therapeutics, Lilly Oncology, Pfizer, Roche/Genentech, Merck, Samsung Bioepis, Tyra Bioscience, and Seattle Genetics; has received research support from Astellas, AstraZeneca, Seattle Genetics, Genentech/Roche, Acrivon, and Lilly Oncology, all of which are outside the submitted work. C.K. Ding has consulted for Intuitive and reports research funding from Bristrol Myers Squibb, all of which are outside the submitted work. S.P. Porten reports research funding from Photocure and Kdx, honoraria from Fergene and CG Oncology, and serves on the steering committee or advisory board for Janssen and Vesica Health, all of which are outside the submitted work, all of which are outside the submitted work. T.W. Friedlander reports research funding from Seagen/Pfizer and Roche/Genetech, and has served in a consulting role for Astellas, Merck, Seagen/Pfizer, Gilead, Bicycle Therapeutics, Atkis Oncology, Bristol Meyers Squibb, and Abbvie, all of which are outside the submitted work. V.S. Koshkin has served in a consulting or advisory role for Astellas, Bicycle Therapeutics, Janssen, EMD Serono, Loxo Oncology, MSD, Seagen/Pfizer; has received institutional research funding from Endocyte/Novartis, Curium, Nektar, Gilead, Taiho, Merck and Seagen/Pfizer; and individual research funding from Eli Lilly, all of which are outside the submitted work. F.Y. Feng reported personal fees from Bluestar Genomics, Astellas, Foundation Medicine, Exact Sciences, Tempus, POINT Biopharma, Janssen, Bayer, Myovant, Roivant, SerImmune, Bristol Meyers Squibb, Novartis, and personal fees from POINT Biopharma and other support from Artera, prior to his death, all of which are outside the submitted work. J.K. Lee holds equity in, is on the scientific advisory board of, and receives research funding from PromiCell Therapeutics, outside the submitted work. He is an inventor on International Patent Application No. PCT/US2023/074229 related to NECTIN4 chimeric antigen receptor T cell therapy. A.P. Wiita reports being an equity holder in Indapta Therapeutics and speaker honoraria from Sanofi and AstraZeneca, all of which are outside the submitted work. J. Chou reports consulting fees from Exai Bio and Bicycle Therapeutics outside the submitted work. K. Chang and J. Chou have filed a provisional patent through the University of California Office of Technology and Management based on this work. All other authors report no disclosures.
Figures

References
-
- Siegel, R. L., Giaquinto, A. N. & Jemal, A. Cancer statistics, 2024. CA: A Cancer J. Clin.74, 12–49 (2024).
-
- Koshkin, V. S. et al. Efficacy of enfortumab vedotin in advanced urothelial cancer: Analysis from the Urothelial Cancer Network to Investigate Therapeutic Experiences (UNITE) study. Cancer128, 1194–1205 (2022). - PubMed
-
- Yu, E. Y. et al. Enfortumab vedotin after PD-1 or PD-L1 inhibitors in cisplatin-ineligible patients with advanced urothelial carcinoma (EV-201): a multicentre, single-arm, phase 2 trial. Lancet Oncol.22, 872–882 (2021). - PubMed
MeSH terms
Substances
Grants and funding
- K12CA260225/U.S. Department of Health & Human Services | NIH | National Cancer Institute (NCI)
- P30CA082103/U.S. Department of Health & Human Services | NIH | National Cancer Institute (NCI)
- K08 CA273514/CA/NCI NIH HHS/United States
- K08CA273514/U.S. Department of Health & Human Services | NIH | National Cancer Institute (NCI)
- P30 CA082103/CA/NCI NIH HHS/United States
LinkOut - more resources
Full Text Sources
Medical

