Loss-of-function variations in solute carrier family 38 member 6 are associated with essential tremor
- PMID: 40931016
- PMCID: PMC12423332
- DOI: 10.1038/s41392-025-02380-y
Loss-of-function variations in solute carrier family 38 member 6 are associated with essential tremor
Abstract
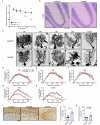
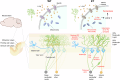
Essential tremor (ET) is a common neurological disease that is characterized by 4-12 Hz kinetic tremors of the upper limbs and high genetic heterogeneity. Although numerous candidate genes and loci have been reported, the etiology of ET remains unclear. A novel ET-related gene was initially identified in a five-generation family via whole-exome sequencing, and other variants were identified in 772 familial ET probands and 640 sporadic individuals via whole-genome sequencing. Among 71 (9.18%) Chinese families and 47 (7.34%) sporadic individuals with ET, we identified 15 types of protein-altering variants in solute carrier family 38 member 6 (SLC38A6), which encodes sodium-coupled neutral amino acid transporter 6 (SNAT6) and is inherited in an autosomal dominant pattern. Over-expression of mutant SNAT6 for the three most common human mutations (p.Y108F, p.M281T and p.G318S) significantly impaired L-arginine (L-Arg) uptake in HeLa cells. The homozygous Slc38a6 deletion mice (Slc38a6-/-) exhibited reduced L-Arg uptake in their cerebellar neurons, tremor, and cerebellar pathology. Slice electrophysiology revealed reduced neuronal Purkinje cell (PC) excitability and elevated inhibitory synaptic transmission in Slc38a6-/- mice, in line with elevated "hairy" basket coverage around the PC soma. Furthermore, heterozygous Slc38a6 deletion (Slc38a6+/-) and PC-specific Slc38a6 deletion (Slc38a6PC-/-) mice also displayed tremor and PC abnormalities similar to those found in Slc38a6-/- mice. These PCs displayed mitochondrial abnormalities and elevated ferroptosis markers (ACSL4, TFRC and Fe ions). In conclusion, we identified variants in SLC38A6 that contribute ~8.35% to ET, generated mouse models displaying tremor, and delineated cerebellar cellular abnormalities and potential mechanisms underlying ET etiology.
© 2025. The Author(s).
Conflict of interest statement
Competing interests: The authors declare no competing interests.
Figures




References
-
- Paulson, G. W. Benign essential tremor: features that aid in diagnosis. Postgrad. Med.71, 105–107 (1982). - PubMed
-
- Jiménez-Jiménez, F. J., Alonso-Navarro, H., García-Martín, E. & Agúndez, J. A. The relationship between Parkinson’s disease and essential tremor: review of clinical, epidemiologic, genetic, neuroimaging and neuropathological data, and data on the presence of cardinal signs of parkinsonism in essential tremor. Tremor. Other Hyperkinet. Mov. (N. Y.)2, tre-02-75–409-3 (2012). - PMC - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

