Plasmapheresis facilitates soluble BCMA clearance and contributes to reversing primary resistance to anti-BCMA immunotherapy in multiple myeloma
- PMID: 40931044
- PMCID: PMC12463651
- DOI: 10.1038/s41375-025-02757-6
Plasmapheresis facilitates soluble BCMA clearance and contributes to reversing primary resistance to anti-BCMA immunotherapy in multiple myeloma
Conflict of interest statement
Competing interests: The authors declare the following competing interests: JMW reports personal fees from Janssen, Sanofi, Takeda, Pfizer, Oncopeptides, Menarini-Stemline, Skyline Dx and GSK, and research support from BMS. KMK reports personal fees from Celgene, BMS, AbbVie, GSK, and Takeda; grants and personal fees from Janssen. HE reports grants and other support from Janssen, BMS, Amgen, GSK, and Sanofi, as well as further support from Takeda and Novartis. LR reports personal fees from BMS, Janssen, Pfizer, Amgen, GSK and Sanofi. All other authors declare no competing interests.
Figures
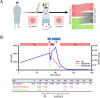
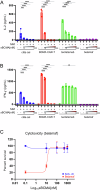
References
-
- Waldschmidt JM, Rasche L, Kortüm KM, Einsele H. Comprehensive review of bispecific antibody constructs in multiple myeloma: affinities, dosing strategies and future perspectives. Clin Lymphoma Myeloma Leuk. 2024;S2152-2650:02423–6. - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

