Structure and mechanism of the mitochondrial calcium transporter NCLX
- PMID: 40931067
- PMCID: PMC12571890
- DOI: 10.1038/s41586-025-09491-0
Structure and mechanism of the mitochondrial calcium transporter NCLX
Abstract
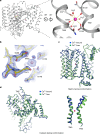
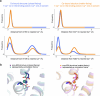
As a key mitochondrial Ca2+ transporter, NCLX regulates intracellular Ca2+ signalling and vital mitochondrial processes1-3. The importance of NCLX in cardiac and nervous-system physiology is reflected by acute heart failure and neurodegenerative disorders caused by its malfunction4-9. Despite substantial advances in the field, the transport mechanisms of NCLX remain unclear. Here we report the cryo-electron microscopy structures of NCLX, revealing its architecture, assembly, major conformational states and a previously undescribed mechanism for alternating access. Functional analyses further reveal an unexpected transport function of NCLX as a H+/Ca2+ exchanger, rather than as a Na+/Ca2+ exchanger as widely believed1. These findings provide critical insights into mitochondrial Ca2+ homeostasis and signalling, offering clues for developing therapies to treat diseases related to abnormal mitochondrial Ca2+.
© 2025. The Author(s).
Conflict of interest statement
Competing interests: The authors declare no competing interests.
Figures












References
-
- De Stefani, D., Rizzuto, R. & Pozzan, T. Enjoy the trip: calcium in mitochondria back and forth. Annu. Rev. Biochem.85, 161–192 (2016). - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

