Neuronal activity-dependent mechanisms of small cell lung cancer pathogenesis
- PMID: 40931074
- PMCID: PMC12571889
- DOI: 10.1038/s41586-025-09492-z
Neuronal activity-dependent mechanisms of small cell lung cancer pathogenesis
Abstract
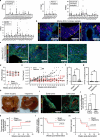
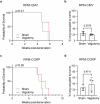
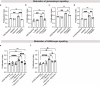
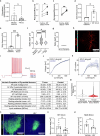
Neural activity is increasingly recognized as a crucial regulator of cancer growth. In the brain, neuronal activity robustly influences glioma growth through paracrine mechanisms1 and by electrochemical integration of malignant cells into neural circuitry via neuron-to-glioma synapses2,3. Outside of the central nervous system, innervation of tumours such as prostate, head and neck, breast, pancreatic, and gastrointestinal cancers by peripheral nerves similarly regulates cancer progression4-12. However, the extent to which the nervous system regulates small cell lung cancer (SCLC) progression, either in the lung or when growing within the brain, is less well understood. SCLC is a lethal high-grade neuroendocrine tumour that exhibits a strong propensity to metastasize to the brain. Here we demonstrate that in the lung, vagus nerve transection markedly inhibits primary lung tumour development and progression, highlighting a critical role for innervation in SCLC growth. In the brain, SCLC cells co-opt neuronal activity-regulated mechanisms to stimulate growth and progression. Glutamatergic and GABAergic (γ-aminobutyric acid-producing) cortical neuronal activity each drive proliferation of SCLC in the brain through paracrine and synaptic neuron-cancer interactions. SCLC cells form bona fide neuron-to-SCLC synapses and exhibit depolarizing currents with consequent calcium transients in response to neuronal activity; such SCLC cell membrane depolarization is sufficient to promote the growth of intracranial tumours. Together, these findings illustrate that neuronal activity has a crucial role in dictating SCLC pathogenesis.
© 2025. The Author(s).
Conflict of interest statement
Competing interests: M.M. holds equity in MapLight Therapeutics and Stellaromics, and stock in CARGO Therapeutics. M.M. was previously on the scientific advisory board for Cygnal Therapeutics. J.S. has equity in and is an advisor for DISCO Pharmaceuticals. B.I. has received consulting fees and honoraria from Volastra Therapeutics Inc., Merck, AstraZeneca, Novartis, Eisai and Janssen Pharmaceuticals and has received research funding to Columbia University from Alkermes, Arcus Biosciences, Checkmate Pharmaceuticals, Compugen, Immunocore, Merck, Regeneron and Synthekine. B.I. is a founder of Basima Therapeutics, Inc.
Figures
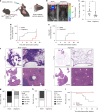
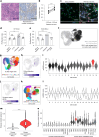

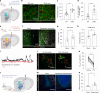
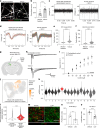








Update of
-
Neuronal-Activity Dependent Mechanisms of Small Cell Lung Cancer Progression.bioRxiv [Preprint]. 2023 Jan 20:2023.01.19.524430. doi: 10.1101/2023.01.19.524430. bioRxiv. 2023. Update in: Nature. 2025 Oct;646(8087):1232-1242. doi: 10.1038/s41586-025-09492-z. PMID: 36711554 Free PMC article. Updated. Preprint.
References
MeSH terms
Substances
Grants and funding
- R01 CA280414/CA/NCI NIH HHS/United States
- R37 NS046579/NS/NINDS NIH HHS/United States
- R01 CA258384/CA/NCI NIH HHS/United States
- DP1 NS111132/NS/NINDS NIH HHS/United States
- R01 NS092597/NS/NINDS NIH HHS/United States
- R01 CA266446/CA/NCI NIH HHS/United States
- R35 CA231997/CA/NCI NIH HHS/United States
- DP2 CA290968/CA/NCI NIH HHS/United States
- P30 CA013696/CA/NCI NIH HHS/United States
- U54 CA274506/CA/NCI NIH HHS/United States
- R00 CA252001/CA/NCI NIH HHS/United States
- P50 CA165962/CA/NCI NIH HHS/United States
- R37 CA258829/CA/NCI NIH HHS/United States
- U19 CA264504/CA/NCI NIH HHS/United States
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases

