Heart failure-specific cardiac fibroblasts contribute to cardiac dysfunction via the MYC-CXCL1-CXCR2 axis
- PMID: 40931092
- PMCID: PMC12436193
- DOI: 10.1038/s44161-025-00698-y
Heart failure-specific cardiac fibroblasts contribute to cardiac dysfunction via the MYC-CXCL1-CXCR2 axis
Abstract
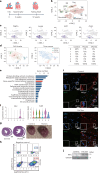
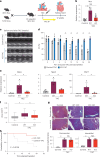
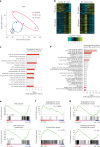
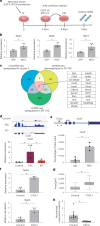
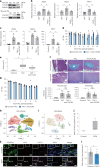
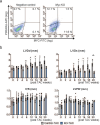
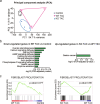
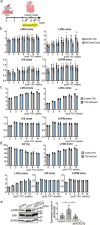
Heart failure (HF) is a growing global health issue. While most studies focus on cardiomyocytes, here we highlight the role of cardiac fibroblasts (CFs) in HF. Single-cell RNA sequencing of mouse hearts under pressure overload identified six CF subclusters, with one specific to the HF stage. This HF-specific CF population highly expresses the transcription factor Myc. Deleting Myc in CFs improves cardiac function without reducing fibrosis. MYC directly regulates the expression of the chemokine CXCL1, which is elevated in HF-specific CFs and downregulated in Myc-deficient CFs. The CXCL1 receptor, CXCR2, is expressed in cardiomyocytes, and blocking the CXCL1-CXCR2 axis mitigates HF. CXCL1 impairs contractility in neonatal rat and human iPSC-derived cardiomyocytes. Human CFs from failing hearts also express MYC and CXCL1, unlike those from controls. These findings reveal that HF-specific CFs contribute to HF via the MYC-CXCL1-CXCR2 pathway, offering a promising therapeutic target beyond cardiomyocytes.
© 2025. The Author(s).
Conflict of interest statement
Competing interests: K.F. is a founding scientist funded by the SAB of Heartseed Co., Ltd. The other authors, J.K., H.H., T. Katsuki, D.K., M. Katoh, T. Ko, M. Ito, M. Katagiri, M. Kubota, S. Yamada, T.N., Y.A., T. Kouka, K.K., M. Kimura, S.I., S.N., I.K., S. Yuasa and M. Ieda, declare no competing interests.
Figures







References
MeSH terms
Substances
Grants and funding
- 22K08925 (S.Y.), 22K08200 (S.Y.), 23K24306 (S.Y.), 24K02452 (S.Y.),/MEXT | Japan Society for the Promotion of Science (JSPS)
- 21J12663 (J.K.), 23KJ0353 (J.K.)/MEXT | Japan Society for the Promotion of Science (JSPS)
- 23K23798 (H.H.)/MEXT | Japan Society for the Promotion of Science (JSPS)
- 22H00471 (S.N.)/MEXT | Japan Society for the Promotion of Science (JSPS)
- 21H05045 (I.K.)/MEXT | Japan Society for the Promotion of Science (JSPS)
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous
