Italian guidelines for cervical cancer screening. Multisocietal recommendations on the use of biomarkers in HPV screening with risk-based approach and GRADE methodology
- PMID: 40931128
- PMCID: PMC12533214
- DOI: 10.1038/s41416-025-03161-8
Italian guidelines for cervical cancer screening. Multisocietal recommendations on the use of biomarkers in HPV screening with risk-based approach and GRADE methodology
Erratum in
-
Correction: Italian guidelines for cervical cancer screening. Multisocietal recommendations on the use of biomarkers in HPV screening with risk-based approach and GRADE methodology.Br J Cancer. 2025 Dec;133(12):1927. doi: 10.1038/s41416-025-03250-8. Br J Cancer. 2025. PMID: 41219391 Free PMC article. No abstract available.
Abstract
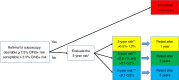
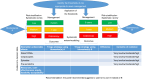
The European Council recommends adopting risk-based screening when relevant. In triaging HPV-positive women, it can be an effective strategy to reduce overtreatment and referral to colposcopy. HPV genotyping and p16/ki67 expression may allow a better risk stratification than cytology. In Italy, recommendations on their use (alone or combined) in screening were developed by a multi-professional (nine scientific societies) and multidisciplinary working group (including patients and decision makers). Grading of Recommendations Assessment, Development and Evaluation (GRADE) Evidence to Decision frameworks were used. Data from large clinical trials on screening populations with long follow-up instructed the biomarkers' evaluation. The working group defined the CIN3+ risk thresholds (a surrogate marker of cancer risk) to guide decisions on management: immediate colposcopy, referral to 1-year and 3-year retesting. The risk-based approach allowed to reduce the number of possible strategies to be compared to five specific healthcare questions framed as PICOs. The prioritised outcomes were risk of cancer and of CIN3+ in HPV+/triage-negative women, number of colposcopies, number of samples to be taken, and number of unneeded treatments. The combination of morphological markers (cytology or p16/ki67) and extended HPV genotyping was the only strategy with a conditional recommendation in favour when compared with cytology.
© 2025. The Author(s), under exclusive licence to Springer Nature Limited.
Conflict of interest statement
Competing interests: SG, FV, FC, and ADM have disclosed no conflicts of interest; PGR participated in the preparation of the IARC Handbook 18 on cervical screening. COIs were collected from all the Contributor Group members. Overall, seven members disclosed potential conflicts of interest and these COIs are included in the final document published on the database of the Italian National System of Guidelines (SNLG) of the National Institute of Health (ISS) (Sistema Nazionale Linee Guida, Istituto Superiore di Sanità): https://www.iss.it/documents/20126/8403884/LG_197_GISCi_Biomarcatori-screening-cervicale_29ago24.pdf/40999ebc-246c-fe99-d483-4da375d9f76a?t=1724836991806 . The Technical Scientific Committee members judged as “potentially relevant” those of three members; they were allowed full participation in the vote, with COI public disclosure. Ethics approval and consent to participate: Not applicable. The guideline development project has been submitted to and approved by the Italian National System of Guidelines (SNLG) of the National Institute of Health (ISS) (Sistema Nazionale Linee Guida, Istituto Superiore di Sanità) https://snlg.iss.it/ .
Figures

References
-
- Ronco G, Confortini M, Maccallini V, Naldoni C, Segnan N, Sideri M, et al. Health Technology Assessment Report. Uso della citologia in fase liquida nello screening dei precursori del cancro del collo uterino [Health technology assessment report. Use of liquid-based cytology for cervical cancer precursors screening]. Epidemiol Prev. 2012;36:e1–33. - PubMed
-
- Zorzi M, Frayle H, Rizzi M, Fedato C, Rugge M, Penon MG, et al. A 3-year interval is too short for re-screening women testing negative for human papillomavirus: a population-based cohort study. BJOG Int J Obstet Gynaecol. 2017;124:1585–93. 10.1111/1471-0528.14575 - DOI

