Genetically modeled GLP1R and GIPR agonism reduce binge drinking and alcohol-associated phenotypes: a multi-ancestry drug-target Mendelian randomization study
- PMID: 40931165
- PMCID: PMC12602350
- DOI: 10.1038/s41380-025-03199-3
Genetically modeled GLP1R and GIPR agonism reduce binge drinking and alcohol-associated phenotypes: a multi-ancestry drug-target Mendelian randomization study
Abstract
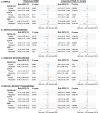
Pharmacological modulation of glucagon-like peptide-1 (GLP-1) and glucose-dependent insulinotropic polypeptide (GIP) through dual GIP/GLP-1 receptor agonists, commonly used for diabetes and obesity, shows promise in reducing alcohol consumption. We applied drug-target Mendelian randomization (MR) using genetic variation at these loci to assess their long-term effects on problematic alcohol use (PAU), binge drinking, alcohol misuse classifications, liver health, and other substance use behaviors. Genetic proxies for lowered BMI, modeling the appetite-suppressing and weight-reducing effects of variants in both the GIPR and GLP1R loci ("GIPR/GLP1R"), were linked with reduced binge drinking in the primary (β = -0.44, 95% CI [-0.72, -0.15], P = 2.42 × 10-3) and replication data (β = -0.13, [-0.22, -0.04], P = 0.0058). HbA1c lowering via GIPR/GLP1R variants was associated with reduced risk of heavy drinking with psychiatric comorbidities versus low-risk drinking (odds ratio [OR] = 0.62, [0.45, 0.85], P = 0.0031), with replication in independent HbA1c data (OR = 0.71, [0.60, 0.84], P = 5.22 × 10-5) and directional consistency with reduced PAU. Analysis of individual loci indicated that both GIPR and GLP1R were protective against heavy drinking, underscoring the importance of both targets. While estimates for other substance use disorders (tobacco, cannabis, opioid) were consistently null, food preference analyses revealed that BMI lowering via GIPR/GLP1R reduced fatty food liking (β = -1.58, [-2.01, -1.14], P = 1.62 × 10-12) and increased vegetarian food liking (β = 2.08, [1.17, 2.99], P = 8.22 × 10-6), implicating metabolic and appetite regulation pathways for the alcohol consumption findings. For liver health, HbA1c lowering via GIPR/GLP1R was associated with reduced NAFLD (β = -0.34, [-0.50, -0.18], P = 2.74 × 10-5) and lower ALT levels (β = -0.26, [-0.38, -0.15], P = 8.39 × 10-6), with replication supporting these findings. Consistency across multiple MR methods and colocalization analyses strengthened causal inference. Mediation analysis suggested reductions in hazardous alcohol consumption partially explain the cardioprotective effects of these agonists. Multi-ancestry analyses supported directionally aligned relationships in non-European cohorts. These findings support further clinical exploration of GLP1R, GIPR, and dual agonists in addiction medicine.
© 2025. This is a U.S. Government work and not under copyright protection in the US; foreign copyright protection may apply.
Conflict of interest statement
Competing interests: The authors declare no competing interests. Ethics considerations: This study used publicly available, de-identified GWASs using participants of European, East Asian, and African ancestries (Table 1). These studies had existing ethical permissions from their respective internal review boards and ethics committees. They included participant informed consent with rigorous quality control for all subjects. No additional informed consent was required in this study. All methods were performed in accordance with the relevant guidelines and regulations.
Figures




References
-
- Substance Abuse and Mental Health Services Administration. Key substance use and mental health indicators in the United States: Results from the 2022 National Survey on Drug Use and Health (HHS Publication No. PEP23-07-01-006, NSDUH Series H-58). 2023. https://www.samhsa.gov/data/report/2022-nsduh-annual-national-report
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

