Developing a Chitosan/polyvinyl alcohol hydrogel for gastro-retentive release of ranitidine and enhanced anti-ulcerative properties
- PMID: 40931332
- PMCID: PMC12421754
- DOI: 10.1186/s12896-025-01040-x
Developing a Chitosan/polyvinyl alcohol hydrogel for gastro-retentive release of ranitidine and enhanced anti-ulcerative properties
Abstract
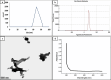
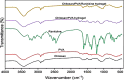
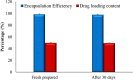
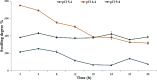
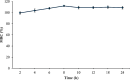
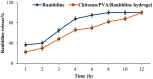
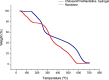
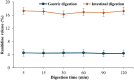
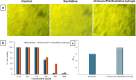
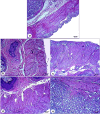
Ranitidine is widely used to treat gastrointestinal conditions, but recent studies have revealed severe potential side effects, including a link to cancer. Therefore, this study aims to develop a new gastro-retentive formulation of ranitidine by utilizing the biocompatibility and biodegradability of Chitosan, along with the strength and hydrophilicity of polyvinyl alcohol (PVA). A chitosan/PVA/ranitidine hydrogel was created using the freeze-thaw method and evaluated for stability, ranitidine release behavior, and efficacy in treating ulcers in rats compared to a commercial formulation. The hydrogel demonstrates an average particle size of 69 nm, a polydispersity index of 0.344, and a zeta potential of + 38 mV. Transmission Electron Microscopy confirmed the spherical shape of the formulation, while X-ray diffraction verified its crystalline structure. Additionally, the study observed an impressive encapsulation efficiency of 98.66% ± 1.01 and a high drug content of 49.82% ± 1.29, as confirmed by Fourier transform infrared analysis. The prepared hydrogel controls the release of ranitidine over 12 h, with an average release of 87.98% ± 4.01%. The hydrogel exhibits minimal degradation over 15 days, greater thermal stability than ranitidine, and adequate stability in acidic gastric conditions. Furthermore, the cytotoxicity assay demonstrated that the hydrogel is biocompatible and promotes cell growth. The study discovered that the hydrogel formulation enhances the effects of ranitidine, particularly its antioxidant and anti-inflammatory properties. In vivo studies illustrated the hydrogel’s promising ulcer-healing properties, suggesting potential use in treating peptic ulcers. Hence, the chitosan/PVA hydrogel can be used as a possible drug delivery system for the sustained release of ranitidine.
Keywords: Chitosan; Gastric ulcer; Gastro-retentive drug delivery; Hydrogel; Ranitidine.
Conflict of interest statement
Declarations. Ethical approval and consent to participate: The experimental protocols and procedures used in this study were approved by the Cairo University Institutional Animal Care and Use Committee (IACUC) in Egypt (Approval no: CU/I/F/5/24). All experimental procedures followed international guidelines for the care and use of laboratory animals. Consent for publication: The authors affirm that no consent for publication was necessary for this study. Competing interests: The authors declare no competing interests.
Figures






References
-
- King FJ, Searle AD, Urquhart MW. Ranitidine—investigations into the root cause for the presence of N-nitroso-N,N-dimethylamine in ranitidine hydrochloride drug substances and associated drug products. Org Process Res Dev. 2020;24:2915–26.
LinkOut - more resources
Full Text Sources
Miscellaneous

