TCA cycle-derived oncometabolites in cancer and the immune microenvironment
- PMID: 40931345
- PMCID: PMC12424225
- DOI: 10.1186/s12929-025-01186-y
TCA cycle-derived oncometabolites in cancer and the immune microenvironment
Abstract
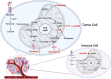
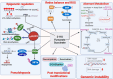
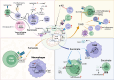
Oncometabolites are aberrant metabolic byproducts that arise from mutations in enzymes of the tricarboxylic acid (TCA) cycle or related metabolic pathways and play central roles in tumor progression and immune evasion. Among these, 2-hydroxyglutarate (2-HG), succinate, and fumarate are the most well-characterized, acting as competitive inhibitors of α-ketoglutarate-dependent dioxygenases to alter DNA and histone methylation, cellular differentiation, and hypoxia signaling. More recently, itaconate, an immunometabolite predominantly produced by activated macrophages, has been recognized for its dual roles in modulating inflammation and tumor immunity. These metabolites influence cancer development through multiple mechanisms, including epigenetic reprogramming, redox imbalance, and post-translational protein modifications. Importantly, their effects are not limited to cancer cells but extend to various components of the tumor microenvironment, such as T cells, macrophages, dendritic cells, and endothelial cells, reshaping immune responses and contributing to immune suppression. In this review, we highlight the emerging insights into the roles of TCA cycle-associated oncometabolites in cancer biology and immune regulation. We discuss how these metabolites impact both tumor-intrinsic processes and intercellular signaling within the tumor microenvironment. Finally, we examine therapeutic strategies targeting oncometabolite pathways, including mutant IDH inhibitors, α-ketoglutarate mimetics, and immunometabolic interventions, with the goal of restoring immune surveillance and improving cancer treatment outcomes.
Keywords: 2-hydroxyglutarate; Epigenetic regulation; Fumarate; Itaconate; Metabolic reprogramming; Oncometabolites; Succinate; TCA cycle; Tumor immunity; α-ketoglutarate.
© 2025. The Author(s).
Conflict of interest statement
Declarations. Ethics approval and consent to participate: Not applicable. Consent for publication: Not applicable. Competing interests: The authors declare no competing interests.
Figures
References
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

