CHOSEN: A Randomized Controlled Feasibility Trial
- PMID: 40932100
- PMCID: PMC12554427
- DOI: 10.1161/JAHA.124.040677
CHOSEN: A Randomized Controlled Feasibility Trial
Abstract
Background: Improving oral health in patients with acutely dysphagic stroke is a plausible approach to prevent pneumonia. We aimed to determine whether a phase 3, definitive trial of oral health care (OHC) treatments, supported by staff education and training, is feasible in stroke unit care.
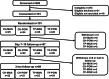
Methods: The CHOSEN (Chlorhexidine or Toothpaste, Manual or Powered Brushing to prevent Pneumonia Complicating Stroke) trial was conducted and reported in line with the Consolidated Standards of Reporting Trials 2010 statement extended to feasibility trials. We aimed to recruit 120 participants with acute stroke and dysphagia within 24 hours of admission, from 4 stroke units in the northwest of England, randomized (1:1:1:1) to 1 of 4 OHC treatments: manual toothbrush or powered toothbrush with either nonfoaming toothpaste or chlorhexidine 1% gel. Stroke unit nursing staff received standardized education and training. Feasibility was assessed using a priori criteria.
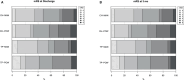
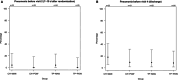
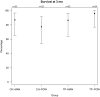
Results: Between January 2022 and end of January 2023, 626 patients were screened. A total of 101 participants (median age, 73 [interquartile range, 62-80] years; median National Institutes of Health Stroke Scale score, 10 [interquartile range, 5-18]; 44% women) were enrolled (77% of eligible patients approached). Adherence was 91%, with no substantial difference between the OHC treatments, and 88% completed follow-up. There were 19 serious adverse events but no marked differences between allocated OHC treatments. In exploratory secondary analyses, again there were no substantial differences in survival, incident pneumonia, modified Rankin Scale score, or quality of life at 3 months between the OHC treatment allocations.
Conclusions: OHC treatments incorporating chlorhexidine and powered brushing and supported by standardized staff training appeared feasible and safe in patients with acutely dysphagic stroke. Progression criteria were met for a definitive trial of efficacy and cost effectiveness.
Registration: URL: https://doi.org/10.1186/ISRCTN52421361; Unique identifier: ISRCTN52421361.
Keywords: chlorhexidine; oral health care; poststroke pneumonia; randomized trial; stroke, acute.
Conflict of interest statement
Oral B provided the powered brushes for the trial but had no other involvement in the trial design, conduct, analyses, or manuscript preparation.
Figures
References
-
- Gittins M, Lobo Chaves M, Vail A, Smith CJ. Does stroke‐associated pneumonia play an important role on risk of in‐hospital mortality associated with severe stroke? A four‐way decomposition analysis of a national cohort of stroke patients. Int J Stroke. 2023;18:1092–1101. doi: 10.1177/17474930231177881 - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

