Optimized Monothiol Thioredoxin Derivative (ORP100S) Protects In Vitro and In Vivo from Radiation and Chemotoxicity Without Promoting Tumor Proliferation
- PMID: 40932638
- PMCID: PMC12622487
- DOI: 10.1002/advs.202504426
Optimized Monothiol Thioredoxin Derivative (ORP100S) Protects In Vitro and In Vivo from Radiation and Chemotoxicity Without Promoting Tumor Proliferation
Abstract
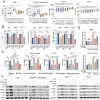
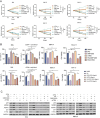
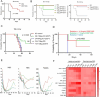
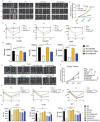
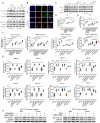
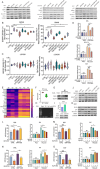
Human thioredoxin-1 (TRX) is a target-selective disulfide reductase with antioxidant, anti-inflammatory, and regulatory functions that mitigates cellular stresses in various organ systems, providing a compelling rationale for therapeutic use as a broad-spectrum cell protectant. However, clinical application of recombinant TRX (rhTRX) is constrained by rapid clearance and proliferative intracellular activity. To overcome these limitations, a rationally designed TRX variant, ORP100S, was engineered for enhanced stability, prolonged extracellular target engagement, and improved protective function, with development of novel single-turnover insulin reduction and hybrid-immunocapture LC-MS assays. ORP100S demonstrates high-yield expression in E. coli (16 g L-1) and exhibits significant in vivo mitigating effects when administered subcutaneously to rodents and non-human primates exposed to otherwise-lethal total-body ionizing radiation. Compared to native TRX, ORP100S displays improved pharmacokinetic and pharmacodynamic properties without promoting murine or human cancer cell proliferation. Additionally, ORP100S protects hematopoietic stem/progenitor cells (HSPCs) from chemotherapy-induced toxicity in vitro and in vivo synergistically with co-administered granulocyte-macrophage colony-stimulating factor (GM-CSF). Mechanistic studies revealed that ORP100S modulates the Kruppel-like factor 4 (KLF4)-p53 pathway to selectively inhibit ferroptosis in HSPCs but not cancer cells. These findings highlight the potential of ORP100S as a novel therapeutic agent for mitigating acute radiation injury and improving the safety and efficacy of chemotherapy without compromising antitumor activity.
Keywords: cell protection; chemoprotection; ferroptosis; hematopoietic stem cells; radiation mitigation; thioredoxin.
© 2025 The Author(s). Advanced Science published by Wiley‐VCH GmbH.
Conflict of interest statement
The authors declare no conflict of interest. OrPro Therapeutics and Duke University are applicants for patents relating to monothiol thioredoxin technology (inventors HM, PH, YK).
Figures


References
MeSH terms
Substances
Grants and funding
- U19AI067798/National Institute of Allergy And Infectious Diseases under Award Number
- R44 HL140735/HL/NHLBI NIH HHS/United States
- UC7 AI180254/AI/NIAID NIH HHS/United States
- UC6 AI058607/AI/NIAID NIH HHS/United States
- U01 AI187020/AI/NIAID NIH HHS/United States
- R43HL114132/National Heart, Lung and Blood Institute grant numbers
- G20-AI167200/National Institutes of Health grant numbers
- U19 AI067798/AI/NIAID NIH HHS/United States
- R43 HL114132/HL/NHLBI NIH HHS/United States
- R21CA280499/National Cancer Institute grant numbers
- R21 CA280499/CA/NCI NIH HHS/United States
- P30CA014236/National Cancer Institute grant numbers
- R21CA234701/National Cancer Institute grant numbers
- R44HL140735/National Heart, Lung and Blood Institute grant numbers
- G20 AI167200/AI/NIAID NIH HHS/United States
- R56HL155582/National Heart, Lung and Blood Institute grant numbers
- U01AI187020/National Institute of Allergy And Infectious Diseases under Award Number
- Cystic Fibrosis Foundation Therapeutics Development Award
- R56 HL155582/HL/NHLBI NIH HHS/United States
- W81XWH2210613/U.S. Department of Defense PRMRP Technology/Therapeutics Development Award
- UC6-AI058607/National Institutes of Health grant numbers
- UC7-AI180254/National Institutes of Health grant numbers
- P30 CA014236/CA/NCI NIH HHS/United States
- R21 CA234701/CA/NCI NIH HHS/United States
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous
