Base mediated approach for the synthesis of deoxybenzoins using γ-aryl-β-ketoesters and benzoyl chlorides
- PMID: 40933092
- PMCID: PMC12418168
- DOI: 10.1039/d5ra05242d
Base mediated approach for the synthesis of deoxybenzoins using γ-aryl-β-ketoesters and benzoyl chlorides
Abstract
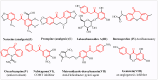
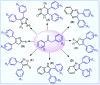
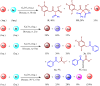
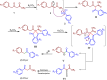
This study introduces a one-pot, transition metal-free strategy for synthesizing deoxybenzoins, overcoming the challenges of conventional methods. The reaction involves dual acylation of γ-aryl β-keto esters using K2CO3 in dioxane at 90 °C, followed by a concerted transformation to form deoxybenzoin. The protocol operates under mild conditions, tolerates a broad range of substrates, and produces minimal by-products, making it a practical, scalable alternative for accessing pharmaceutically relevant deoxybenzoins.
This journal is © The Royal Society of Chemistry.
Conflict of interest statement
The authors declare no competing financial interest.
Figures



References
-
- Preininger V. Šimánek V. Gašić O. Šantavý F. Dolejš L. Phytochemistry. 1973;12:2513–2515.
- Lamblin M. Couture A. Deniau E. Grandclaudon P. Org. Biomol. Chem. 2006;5:1466–1471. - PubMed
- Rey J.-P. Levesque J. Pousset J.-L. Roblot F. J. Chromatogr. A. 1991;587:314–317.
- Boonyaketgoson S. Rukachaisirikul V. Phongpaichit S. Trisuwan K. Phytochem. Lett. 2019;31:96–100.
- Driowya M. Saber A. Marzag H. Demange L. Bougrin K. Benhida R. Molecules. 2016;21:1032. - PMC - PubMed
- Kaufmann D. Fünfschilling P. C. Beutler U. Hoehn P. Lohse O. Zaugg W. Tetrahedron Lett. 2004;45:5275–5278.
- Haasio K., in International Review of Neurobiology, ed. E. Nissinen, Academic Press, 2010, pp. 163–189 - PubMed
- Luo Y. Li H.-Q. Zhou Y. Li Z.-L. Yan T. Zhu H.-L. ChemMedChem. 2010;5(7):1110–1116. - PubMed
-
- Fokialakis N. Lambrinidis G. Mitsiou D. J. Aligiannis N. Mitakou S. Skaltsounis A.-L. Pratsinis H. Mikros E. Alexis M. N. Chem. Biol. 2004;11:397–406. - PubMed
- Xiao Z.-P. Shi D.-H. Li H.-Q. Zhang L.-N. Xu C. Zhu H.-L. Bioorg. Med. Chem. 2007;15:3703–3710. - PubMed
- Li H.-Q. Xue J.-Y. Shi L. Gui S.-Y. Zhu H.-L. Eur. J. Med. Chem. 2008;43:662–667. - PubMed
- Li H.-Q. Luo Y. Lv P.-C. Shi L. Liu C.-H. Zhu H.-L. Bioorg. Med. Chem. Lett. 2010;20:2025–2028. - PubMed
-
- Moffett R. B. Strube R. E. Skaletzky L. J. Med. Chem. 1971;14:1088–1100. - PubMed
- Zou Y. Zhang S. Wen X. Wang G. Sun Y. Liu S. Peng T. Gao Y. Wang L. Tetrahedron Lett. 2017;58:2835–2837.
- Ellzey K. A. Ranganathan T. Zilberman J. Coughlin E. B. Farris R. J. Emrick T. Macromolecules. 2006;39:3553–3558.
-
- Longstreet A. R. Jo M. Chandler R. R. Hanson K. Zhan N. Hrudka J. J. Mattoussi H. Shatruk M. McQuade D. T. J. Am. Chem. Soc. 2014;136:15493–15496. - PubMed
- Torán R. Vila C. Sanz-Marco A. Muñoz M. C. Pedro J. R. Blay G. Eur. J. Org Chem. 2020;2020:627–630.
- Gao J. Ren Z.-G. Lang J.-P. Chin. Chem. Lett. 2017;28:1087–1092.
- Fernandes A. J. Giri R. Houk K. N. Katayev D. Angew. Chem., Int. Ed. 2024;63:e202318377. - PubMed
- Wang X. Qi F. Jiang Z. Yan M. Xu L. Dyes Pigm. 2021;186:108999.
- Zhu X. Guo R. Zhang X. Gao Y. Jia Q. Wang Y. Adv. Synth. Catal. 2020;362(15):3190–3201.
-
- Roslan I. I. Ng K.-H. Wu J.-E. Chuah G.-K. Jaenicke S. J. Org. Chem. 2016;81(19):9167–9174. - PubMed
- Roslan I. I. Ng K.-H. Chuah G.-K. Jaenicke S. Eur. J. Org Chem. 2017;2017:704–709.
- Mao S. Zhu X.-Q. Gao Y.-R. Guo D.-D. Wang Y.-Q. Chem. - Eur. J. 2015;21:11335–11339. - PubMed
- Wang Z.-Y. Liu Q. Wang K.-K. Liu M. Han Y. Sun A. Ma X. Asian J. Org. Chem. 2021;10:766–770.
- Shashank A. B. Karthik S. Madhavachary R. Ramachary D. B. Chem. - Eur. J. 2014;20:16877–16881. - PubMed
- Zhang C. Xu Z. Zhang L. Jiao N. Tetrahedron. 2012;68:5258–5262.
- Shen Z.-L. Xu X.-P. Ji S.-J. J. Org. Chem. 2010;75:1162–1167. - PubMed
- Potukuchi H. K. Spork A. P. Donohoe T. J. Org. Biomol. Chem. 2015;13:4367–4373. - PMC - PubMed
- Sivanandan S. T. Shaji A. Ibnusaud I. Seechurn C. C. C. J. Colacot T. J. Eur. J. Org Chem. 2015;2015:38–49.
LinkOut - more resources
Full Text Sources

