Oviduct epithelium interactions: roles in sperm selection and embryo quality
- PMID: 40933865
- PMCID: PMC12419264
- DOI: 10.1590/1984-3143-AR2025-0035
Oviduct epithelium interactions: roles in sperm selection and embryo quality
Abstract
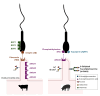
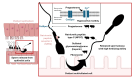
This review provides an up-to-date overview of the roles of the oviduct during the periconception period and underlying mechanisms. The functions of the oviduct before, during, and after fertilization are highlighted, with special focus on the effects of epithelial cell contact and luminal secretions on sperm selection mechanisms and acquisition of fertilization ability. The current knowledge on how the oviduct contributes to support fertilization and embryo development via the overall physical milieu (oxygen tension, fluid current, ciliated epithelial cells) and the role of its secretions is also provided. Altogether, the review underlines the unique role of the oviduct during gamete selection and early embryo development, which so far has not been completely possible to mirror when assisted reproductive technologies (ART) are used. Unveiling the most important functional components of oviductal secretions that contribute to better sperm selection, and boost sperm fertilizing ability and early embryo development, can indeed be useful to improve the outcomes of current in vitro systems used in ART.
Keywords: embryo; fallopian tube; gamete; oviduct; spermatozoa.
Copyright © The Author(s).
Conflict of interest statement
Conflicts of interest: The authors have no conflict of interest to declare.
Figures



References
LinkOut - more resources
Full Text Sources
