Drug hypersensitivity reactions in children in clinical practice: A WAO Statement
- PMID: 40933958
- PMCID: PMC12419032
- DOI: 10.1016/j.waojou.2025.101087
Drug hypersensitivity reactions in children in clinical practice: A WAO Statement
Abstract
Drug hypersensitivity reactions (DHRs) in children and adolescents are less common than in adults but can have serious consequences if mismanaged. Mislabeling children as drug-allergic due to incomplete diagnostic evaluations leads to unnecessary medication restrictions, increased healthcare costs, and suboptimal treatment choices. This Statement from the World Allergy Organization (WAO) provides evidence-based recommendations for evaluating and managing pediatric DHRs, emphasizing accurate diagnosis through in vivo and in vitro testing, risk stratification, and personalized approaches. Antibiotics, particularly β-lactams, and non-steroidal anti-inflammatory drugs (NSAIDs) are the most frequently implicated drugs, with non-immediate reactions, such as maculopapular exanthema, being the most common presentation. The document also addresses emerging concerns, including monoclonal antibody-induced anaphylaxis and drug-induced enterocolitis syndrome. It underscores the need for specialized care in allergy centers with expertise in pediatric populations and advocates for multidisciplinary programs to manage complex cases, such as chemotherapy hypersensitivity and perioperative drug allergy. By addressing diagnostic challenges and clinical uncertainties, this document aims to improve the management of DHRs in children, reduce mislabeling, and enhance patient outcomes worldwide.
© 2025 The Author(s).
Conflict of interest statement
The authors report no competing interests with regards to this document.
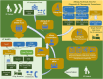
Figures

References
-
- Sousa-Pinto B., Almeida-Fonseca J., Rebelo Gomes E. Frequency of self-reported drug allergy: a systematic review and meta-analysis with meta-regression. Ann Allergy Asthma Immunol. 2017 Oct;119(4):362–373.e2. - PubMed
-
- Capanoglu M., Erkocoglu M., Kaya A., et al. Confirmation of drug allergy in a general pediatrics outpatient clinic. Ann Allergy Asthma Immunol. 2022 Dec;129(6):784–789. - PubMed
-
- Jones T.W., Fino N., Olson J., Hersh A.L. The impact of beta-lactam allergy labels on hospitalized children. Infect Control Hosp Epidemiol. 2021 Mar;42(3):318–324. - PubMed
Publication types
LinkOut - more resources
Full Text Sources

