Organosilicon Precursors for Efficient Aromatic Copper-Mediated Radiocyanation
- PMID: 40934031
- PMCID: PMC12419773
- DOI: 10.1016/j.chempr.2025.102707
Organosilicon Precursors for Efficient Aromatic Copper-Mediated Radiocyanation
Abstract
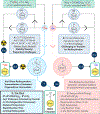
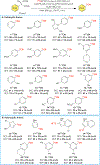
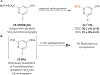
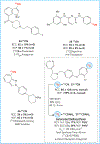
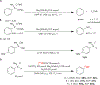
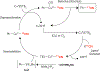
Copper-mediated radiolabelling has transformed how (hetero)aromatic imaging agents are prepared for positron emission tomography (PET). However, current methods maintain critical stability, reactivity, and toxicity concerns paramount for safely and reproducibly generating radiomedicines. To overcome these limitations, a copper-mediated 11C-cyanation reaction is presented that leverages heptamethyltrisiloxanes as (hetero)aryl nucleophiles. Rapid ipso-radiocyanation occurs in conversions surpassing related precursors, while offering stability and safety advantages. Multiple bioactive scaffolds relevant to (pre)clinical PET were labelled to showcase the broader significance of this protocol. Most notably, an automated radiosynthesis of a κ-opioid receptor antagonist currently used in clinical PET studies is reported using this method. Overall, adopting aryl silane precursors will improve radiochemical space and support the production of PET nuclear medicines.
Keywords: Carbon-11; Copper-Mediated Radiolabelling; Nuclear Medicine; Organosilane; Positron Emission Tomography; Radiochemistry; Radiocyanation.
Conflict of interest statement
DECLARATION OF INTERESTS The authors declare no competing interests.
Figures

References
-
- Deng X, Rong J, Wang L, Vasdev N, Zhang L, Josephson L, and Liang SH (2019). Chemistry for Positron Emission Tomography: Recent Advances in 11C-, 18F-, 13N-, and 15O-Labeling Reactions. Angew. Chem. Int. Ed. 58, 2580–2605. 10.1002/anie.201805501. - DOI
Grants and funding
LinkOut - more resources
Full Text Sources
