Neutralizing antibody responses over time in a demographically and clinically diverse cohort of individuals recovered from SARS-CoV-2 acquisition in Africa: A cohort study
- PMID: 40934225
- PMCID: PMC12425307
- DOI: 10.1371/journal.pgph.0005156
Neutralizing antibody responses over time in a demographically and clinically diverse cohort of individuals recovered from SARS-CoV-2 acquisition in Africa: A cohort study
Abstract
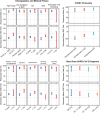
COVID-19 has affected millions worldwide. Research characterized immune responses of individuals who acquired SARS-CoV-2 and identified co-factors, such as HIV, associated with greater likelihood of poor clinical outcomes. SARS-CoV-2-specific neutralizing antibodies (nAbs) are a strong correlate of protection but their elicitation in people living with HIV (PLWH), and particularly in southern Africa, is less well characterized. HVTN 405/HPTN 1901 was an observational cohort study of individuals recently recovered from SARS-CoV-2. We describe 323 participants enrolled early in the pandemic (June 2020 to January 2021) in Zambia (n = 12), Malawi (n = 13), Zimbabwe (n = 59), and South Africa (n = 239), profiling their SARS-CoV-2-specific nAb responses and associations with demographics, comorbidities, disease severity, and time since diagnosis based on linear and logistic regression. Participants' median age was 39 years, 63.5% were assigned female sex at birth, 71.2% were black African, and 39 (12.1%) were PLWH. Approximately one in four participants (25.7%) had asymptomatic SARS-CoV-2, 47.4% were symptomatic but not hospitalized, and 26.9% were hospitalized with COVID-19. Participants in these groups were enrolled at a median of 51.5 days, 53 days, and 60 days post-SARS-CoV-2 diagnosis, respectively. SARS-CoV-2 nAbs were measured in serum using one of two calibrated assays. Most (291/322, 90.4%) participants had positive nAb responses at enrollment. Across all participants, nAb responses generally declined in magnitude between enrollment and 2-3 months thereafter, then increased through month 12 coincident with epidemiologically observed new waves of acquisition. In a multivariate model adjusted for potentially confounding factors, PLWH had a 65% lower geometric mean (GM) nAb ID50 titer compared to people without HIV (PWOH) (GMR: 0.35, p = 0.003, q = 0.006). Greater disease severity, older age (>55 years), high BMI (≥30) and diabetes were associated with higher nAb ID50 titers (all p < 0.05, all q < 0.20).These findings are important, as nAb titers are predictive of vulnerability to COVID-19.
Copyright: This is an open access article, free of all copyright, and may be freely reproduced, distributed, transmitted, modified, built upon, or otherwise used by anyone for any lawful purpose. The work is made available under the Creative Commons CC0 public domain dedication.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures


References
-
- World Health Organization. WHO Coronavirus (COVID-19) dashboard [cited 2024 September 18]. Available from: https://data.who.int/dashboards/covid19/cases
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous
