Differentiating clinically important interstitial lung abnormalities in lung cancer screening
- PMID: 40935564
- PMCID: PMC12506105
- DOI: 10.1136/bmjresp-2025-003298
Differentiating clinically important interstitial lung abnormalities in lung cancer screening
Abstract
Background: Interstitial lung abnormalities (ILAs) are common incidental findings in lung cancer screening (LCS). However, challenges remain in identifying clinically relevant ILAs as highlighted in a joint statement by a European multidisciplinary task force led by the European Respiratory Society (ERS). To address these challenges, we analysed ILAs identified in one of Europe's largest LCS studies.
Methods: Of 11 635 LCS individuals, 417 screen-detected ILAs were evaluated using a new visual classification system focused on traction bronchiolectasis: non-fibrotic ILA (no traction bronchiolectasis), fibrotic ILA (traction bronchiolectasis in ≤2 lobes); undiagnosed interstitial lung disease (traction bronchiolectasis in >2 lobes). Observer agreement was compared with Fleischner Society ILA classification using Cohen's Kappa. An age, sex and smoking history-matched control group allowed the examination of associations between baseline ILA/UILD and comorbidities, forced vital capacity (FVC), hospitalisations (Student's t-tests) and mortality (univariable and multivariable Cox proportional hazards models).
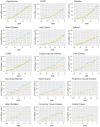
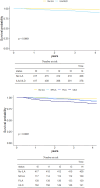
Findings: Our visual ILA classification showed superior interobserver agreement (K=0.76) versus the Fleischner ILA classification (K=0.64). ILA/UILD subjects had more prevalent comorbidities, increasing (vs controls) approximately 10 years prior to ILA/UILD diagnosis. Compared with controls, mortality rates were 6-fold higher for UILD participants and 3-fold higher for fibrotic and non-fibrotic ILA subtypes. On multivariable Cox regression analysis, ILA/UILD presence (HR=4.90, 95% CI =2.36 to 10.10, p<0.001) showed stronger independent associations with mortality than baseline FVC (HR=0.98, 95% CI =0.96 to 1.00, p=0.04).
Conclusion: We demonstrate a new reproducible classification of clinically important ILA/UILDs in LCS populations. We highlight that FVC shows limited associations with mortality in ILA/UILD subjects. Increased multiorgan comorbidity in ILA/UILD subjects highlights a need for comprehensive early multisystem evaluation.
Keywords: Idiopathic Pulmonary Fibrosis; Imaging/CT MRI etc; Interstitial Fibrosis.
© Author(s) (or their employer(s)) 2025. Re-use permitted under CC BY. Published by BMJ Group.
Conflict of interest statement
Competing interests: SUMMIT is sponsored and conducted by University College London and funded by GRAIL through a research grant awarded to SMJ as principal investigator. AN is a member of the advisory board for Aidence BV and Faculty Science, has received a consultation fee from MSD and honorarium for travel to a conference from Takeda. AN is an Executive Committee member for the British Society of Thoracic Imaging, Lung Taskforce member for the British Lung Foundation and clinical lead for the NHS England Targeted Lung Health Checks Programme. AH has received an honorarium for an advisory board meeting for GRAIL, a consultation fee for Evidera Inc for a GRAIL initiated project, and previously owned shares in Illumina. SMJ has received honoraria for travel, consultancy or speaking from Astra Zeneca, BARD1 Bioscience, Optellum, Jansen, Takeda, Evidera and Achilles Therapeutics. SMJ received grant funding from Owlstone for a separate research study and has a family member who is an employee of Astra Zeneca. JJ has received grant funding from GlaxoSmithKline, Wellcome Trust, Gilead and Microsoft Research and consultation fees from Boehringer Ingelheim, F. Hoffmann-La Roche, GlaxoSmithKline, NHSX and Takeda. GJ has received grants and contracts with Astra Zeneca, Galecto, GlaxoSmithKline, Nordic Biosciences, RedX, Pliant. GJ has received consultation fees from Apollo Therapeutics, Astra Zeneca, Brainomix, Bristol Myers Squibb, Chiesi, Cohbar, Galecto, GlaxoSmithKline, Roche, Resolution Therapeutics and Pliant. GJ has received payment for expert testimony by Pinsent Masons LLP. GJ participates on a Data Safety Monitoring Board or Advisory Board for Boehringer Ingelheim, Galapagos and Vicore. GJ is on the board for NuMedii and is president for Action for Pulmonary Fibrosis. GJ is the chair of the editorial board for BMJ Open Respiratory Research. JJ is involved in the following patent applications (UK patent application numbers 2113765.8 and GB2211487.0). AC received a HEE NIHR Pre-Doctoral Clinical Academic Fellowship. All authors perceive that these disclosures pose no academic conflict for this study and declare no other relationships or activities that could appear to have influenced the submitted work. AD has received consulting fees from Boehringer Ingelheim, Roche, Astra Zeneca, Brainomix and has stock in Brainomix. NN has received consulting fees from Amgen, Astra Zeneca, Boehringer Ingelheim, Bristol Myers Squibb, Daiichi Sankyo, EQRx, Fujifilm, Guardant Health, Intuitive, Janssen, Lilly, Merck Sharp & Dohme, Olympus, and Roche. NN has received honorarium for travel, consultancy or speaking from Astra Zeneca, Bristol Myers Squibb, Fujifilm, Intuitive, Janssen, Lilly, Merck Sharp & Dohme, Olympus, Sanofi and Roche. NN is in the Steering committee British Thoracic Oncology Group, Director of UK Lung Cancer Coalition and Senior Clinical Lead of National Lung Cancer Audit. JP has received consulting fees from Istesso. All authors perceive that these disclosures pose no academic conflict for this study and declare no other relationships or activities that could appear to have influenced the submitted work.
Figures

References
-
- Dickson JL, Hall H, Horst C, et al. Uptake of invitations to a lung health check offering low-dose CT lung cancer screening among an ethnically and socioeconomically diverse population at risk of lung cancer in the UK (SUMMIT): a prospective, longitudinal cohort study. Lancet Public Health. 2023;8:e130–40. doi: 10.1016/S2468-2667(22)00258-4. - DOI - PMC - PubMed
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
