Effect of methylcobalamin on capecitabine induced hand-foot syndrome in patients with HER2 negative early breast cancer: multicentre, double blind, randomised, placebo controlled, phase 3 trial
- PMID: 40935571
- PMCID: PMC12423938
- DOI: 10.1136/bmj-2025-084290
Effect of methylcobalamin on capecitabine induced hand-foot syndrome in patients with HER2 negative early breast cancer: multicentre, double blind, randomised, placebo controlled, phase 3 trial
Abstract
Objective: To evaluate whether methylcobalamin could effectively and safely prevent hand-foot syndrome in patients with human epidermal growth factor receptor 2 (HER2) negative early breast cancer receiving adjuvant capecitabine treatment.
Design: Multicentre, double blind, randomised, placebo controlled, phase 3 trial.
Setting: Seven hospitals in China between January 2022 and February 2024.
Participants: Women aged 18-75 years with pathologically confirmed HER2 negative early breast cancer who were scheduled to receive adjuvant capecitabine treatment.
Interventions: Eligible patients were randomly assigned in a 1:1 ratio to receive methylcobalamin at a dose of 0.5 mg orally, three times daily, or a placebo for a maximum of 24 weeks.
Main outcome measures: The primary endpoint was the incidence of grade ≥2 hand-foot syndrome occurring for the first time during capecitabine treatment in the intention-to-treat analysis.
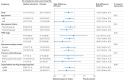
Results: 234 patients were randomly assigned to receive methylcobalamin (n=117) or placebo (n=117) and were included in the intention-to-treat and safety analysis. Grade ≥2 hand-foot syndrome occurred in 17 (14.5%) of 117 patients in the methylcobalamin group and 34 (29.1%) of 117 patients in the placebo group (risk difference -14.5%, 95% confidence interval -24.9% to -4.1%; one sided P value=0.003). The rate of reduction or discontinuation of capecitabine treatment because of hand-foot syndrome was 7.7% (9 of 117) in the methylcobalamin group and 13.7% (16 of 117) in the placebo group (risk difference -6.0%, 95% confidence interval -13.9% to 1.9%). The two groups showed similar incidence of any other adverse events (88 (75.2%) in the methylcobalamin group and 95 (81.2%) in the placebo group). No methylcobalamin specific adverse events were observed.
Conclusions: Oral methylcobalamin significantly lowered the severity of hand-foot syndrome by reducing the incidence of grade ≥2 symptoms without unexpected safety concerns in women with HER2 negative early breast cancer who were receiving adjuvant capecitabine treatment. The findings support the use of methylcobalamin to prevent capecitabine associated severe hand-foot syndrome in this patient population.
Trial registration: ClinicalTrials.gov NCT05165069.
© Author(s) (or their employer(s)) 2019. Re-use permitted under CC BY-NC. No commercial re-use. See rights and permissions. Published by BMJ.
Conflict of interest statement
Competing interests: All authors have completed the ICMJE uniform disclosure form at https://www.icmje.org/disclosure-of-interest/ and declare: support from National Science and Technology Major Project for the Prevention and Treatment of Cancer, Cardiovascular, Respiratory, and Metabolic Diseases, Noncommunicable Chronic Diseases-National Science and Technology Major Project of China, Fundamental Research Funds for the Central Universities, Sun Yat-sen University, Sun Yat-sen University Clinical Research 5010 Program, National Natural Science Foundation of China, Science and Technology Projects in Guangzhou, Guangdong Science and Technology Department, Project of The Beijing Xisike Clinical Oncology Research Foundation, Department of Natural Resources of Guangdong Province, the Science and Technology Planning Project of Guangdong Province, Guangzhou Science and Technology Program, High-tech, Major and Characteristic Technology Projects in Guangzhou Area, Guangdong Provincial Clinical Research Centre for Breast Diseases, and National Key R&D Program of China for the submitted work; no financial relationships with any organisations that might have an interest in the submitted work in the previous three years; no other relationships or activities that could appear to have influenced the submitted work.
Figures
References
-
- Wang X, Wang SS, Huang H, et al. South China Breast Cancer Group (SCBCG) . Effect of capecitabine maintenance therapy using lower dosage and higher frequency vs observation on disease-free survival among patients with early-stage triple-negative breast cancer who had received standard treatment: the SYSUCC-001 randomized clinical trial. JAMA 2021;325:50-8. 10.1001/jama.2020.23370. - DOI - PMC - PubMed
-
- Hong RX, Xu F, Xia W, et al. Metronomic capecitabine plus aromatase inhibitor as initial therapy in patients with hormone receptor-positive, human epidermal growth factor receptor 2-negative metastatic breast cancer: the phase III MECCA trial. J Clin Oncol 2025;43:1314-24. 10.1200/JCO.24.00938. - DOI - PMC - PubMed
-
- Janusch M, Fischer M, Marsch W, et al. The hand-foot syndrome—a frequent secondary manifestation in antineoplastic chemotherapy. Eur J Dermatol 2006;16:494-9. - PubMed
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous