Sorafenib-to-regorafenib sequence-induced atherosclerotic cardiovascular disease: a novel case report
- PMID: 40936714
- PMCID: PMC12420242
- DOI: 10.3389/fonc.2025.1615472
Sorafenib-to-regorafenib sequence-induced atherosclerotic cardiovascular disease: a novel case report
Abstract
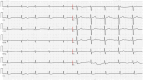
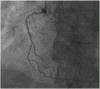
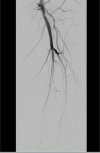
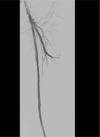
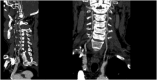
This case report describes a 59-year-old male with HBV-associated hepatocellular carcinoma who developed progressive atherosclerotic cardiovascular disease (ASCVD) during sequential treatment with sorafenib and regorafenib. Initial sorafenib therapy (400 mg twice daily) led to hand-foot syndrome, necessitating dose reduction, and subsequently resulted in the onset of hypertension (152/92 mmHg), proteinuria (3+), and microscopic hematuria (2+). Due to disease progression, the patient was transitioned to regorafenib (160 mg daily), during which time he experienced worsening vascular toxicity manifested as lower extremity arterial occlusion, non-ST elevation myocardial infarction, and cerebral ischemia. Angiography revealed critical multivessel coronary disease (95-99% left anterior descending artery [LAD] stenosis) and complete femoropopliteal occlusion, requiring revascularization procedures. Notably, these severe ASCVD manifestations occurred despite a low baseline cardiovascular risk (10-year ASCVD risk of 4.5%) and the absence of traditional risk factors, underscoring the cumulative atherogenic effects of sequential vascular endothelial growth factor (VEGF) pathway inhibition. This case highlights the importance of continuous cardiovascular monitoring during tyrosine kinase inhibitor therapy, particularly when transitioning between agents.
Keywords: atherosclerotic cardiovascular disease; drug-related toxicity; hepatocellular carcinoma; regorafenib; sorafenib; tyrosine kinase inhibitors.
Copyright © 2025 Xu, Quan, Guo, Liu, Lu, Yang, Xu, Jin, Qu and Ji.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures



References
Publication types
LinkOut - more resources
Full Text Sources

