The Inhibitory Effect of Niosome Containing Myrtenol as an Innovative Approach to Combat Methicillin-Resistant Staphylococcus aureus (MRSA)
- PMID: 40937047
- PMCID: PMC12422854
- DOI: 10.1155/cjid/2742569
The Inhibitory Effect of Niosome Containing Myrtenol as an Innovative Approach to Combat Methicillin-Resistant Staphylococcus aureus (MRSA)
Abstract
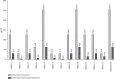
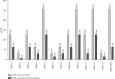
The clinical challenge of staphylococcal treatment is increasing globally, making it critical to find effective strategies to hinder the spread of resistant isolates, particularly methicillin-resistant Staphylococcus aureus (MRSA). Niosomal drug delivery systems, known for their controlled release profiles and other advantageous features, can enhance the efficacy of antimicrobial ability of loaded agents. This study aims to propose a novel drug delivery system for combating staphylococcal resistance challenge through examining the antibacterial and antibiofilm properties of myrtenol-loaded niosomal system against MRSA isolates. The niosomal formulation was prepared using the thin-film hydration process, and its physicochemical characteristics were assessed through entrapment efficiency (EE %), in vitro release profile, field-emission scanning electron microscopy (FE-SEM), and dynamic light scattering (DLS). In addition, minimum inhibitory concentrations (MICs) and minimum bactericidal concentrations (MBCs) were measured and compared with free myrtenol to evaluate the anti-MRSA activity of the formulated niosomal myrtenol. Furthermore, the effectiveness of niosome containing myrtenol against MRSA biofilms was investigated by examining biofilm minimum inhibitory and eradication concentrations (BMIC/BMEC). Additionally, the cytotoxicity of synthesized niosomes was assessed on the human foreskin fibroblast cell line (HFF). Results from FE-SEM showed that myrtenol-loaded niosomes were spherical with a diameter of 122.1 nm, while the hydrodynamic size reported from DLS was 130.8 nm. The surface charge and EE% of the prepared niosomal formulation were -53.6 mV and 62.90%, respectively. The niosomal myrtenol formulation demonstrated the increased antibacterial activity in comparison with free myrtenol formulation. Furthermore, myrtenol-loaded niosomes reduced the biofilm formation potential in all MRSA isolates and effectively eradicated bacterial biofilms at equivalent concentrations of the non-niosomal formulation. According to this study, niosomes exhibit high potential for drug delivery due to several favorable characteristics, including a sustained-release profile, nontoxicity, small size, and high EE%. Niosomal delivery system presents a novel approach to combat bacterial infections, particularly those caused by MRSA isolates by enhancing antibacterial and antibiofilm activities of free myrtenol.
Keywords: biofilm; drug delivery system; methicillin-resistant Staphylococcus aureus (MRSA); myrtenol; niosome; resistant wound infection.
Copyright © 2025 Jaber Hemmati et al. Canadian Journal of Infectious Diseases and Medical Microbiology published by John Wiley & Sons Ltd.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures





References
-
- Kyaw M. H., Kern D. M., Zhou S., Tunceli O., Jafri H. S., Falloon J. Healthcare Utilization and Costs Associated With S. aureus and P. aeruginosa Pneumonia in the Intensive Care Unit: A Retrospective Observational Cohort Study in a US Claims Database. BMC Health Services Research . 2015;15(1):p. 241. doi: 10.1186/s12913-015-0917-x. - DOI - PMC - PubMed
-
- Tocco I., Zavan B., Bassetto F., Vindigni V. Nanotechnology-Based Therapies for Skin Wound Regeneration. Journal of Nanomaterials . 2012;2012(1):p. 714134. doi: 10.1155/2012/714134. - DOI
-
- Hosseini M., Shapouri Moghaddam A., Derakhshan S., et al. Correlation Between Biofilm Formation and Antibiotic Resistance in MRSA and MSSA Isolated From Clinical Samples in Iran: A Systematic Review and Meta-Analysis. Microbial Drug Resistance . 2020;26(9):1071–1080. doi: 10.1089/mdr.2020.0001. - DOI - PubMed
LinkOut - more resources
Full Text Sources

