Multimaterial Shape Memory Polymer Fibers for Advanced Drug Release Applications
- PMID: 40937145
- PMCID: PMC12420707
- DOI: 10.1007/s42765-025-00571-4
Multimaterial Shape Memory Polymer Fibers for Advanced Drug Release Applications
Abstract
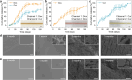
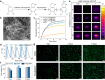
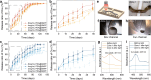
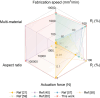
Stimuli-responsive polymers offer unprecedented control over drug release in implantable delivery systems. Shape memory polymer fibers (SMPFs), with their large specific surface area and programmable properties, present promising alternatives for triggerable drug delivery. However, the existing SMPFs face limitations in resolution, architecture, scalability, and functionality. We introduce thermal drawing as a materials and processing platform to fabricate microstructured, multimaterial SMPFs that are tens of meters long, with high resolution (10 μm) and extreme aspect ratios (> 105). These novel fibers achieve highly controlled, sequential drug release over tailored time periods of 6 months. Post thermal drawing photothermal coatings enable accelerated, spatially precise drug release within 4 months and facilitate light-triggered, untethered shape recovery. The fibers' fast self-tightening capability within 40 s shows their potential as smart sutures for minimally invasive procedures that deliver drugs simultaneously. In addition, the advanced multimaterial platform facilitates the integration of optical and metallic elements within SMP systems, allowing highly integrated fibers with shape memory attributes and unprecedented functionalities. This versatile technology opens new avenues for diverse biomedical applications, including implantable drug delivery systems, smart sutures, wound dressings, stents, and functional textiles. It represents a significant advancement in precise spatio-temporal control of drug delivery and adaptive medical devices.
Supplementary information: The online version contains supplementary material available at 10.1007/s42765-025-00571-4.
Keywords: Drug delivery; Multifunctionality; Multimaterial fibers; Sequential drug release; Shape memory polymers.
© The Author(s) 2025.
Conflict of interest statement
Conflict of interestThe authors declare no conflict of interest.
Figures



References
-
- He GQ, Li H, Liu J, Hu YL, Liu Y, Wang ZL, Jiang P. Recent progress in implantable drug delivery systems. Adv Mater. 2024;36:2312530. - PubMed
-
- Talebian S, Foroughi J, Wade SJ, Vine KL, Dolatshahi-Pirouz A, Mehrali M, Conde J, Wallace GG. Biopolymers for antitumor implantable drug delivery systems: recent advances and future outlook. Adv Mater. 2018;30:1706665. - PubMed
-
- Kutner N, Kunduru KR, Rizik L, Farah S. Recent advances for improving functionality, biocompatibility, and longevity of implantable medical devices and deliverable drug delivery systems. Adv Funct Mater. 2021;31:2010929.
-
- Stuart MAC, Huck WTS, Genzer J, Müller M, Ober C, Stamm M, Sukhorukov GB, Szleifer I, Tsukruk VV, Urban M, Winnik F, Zauscher S, Luzinov I, Minko S. Emerging applications of stimuli-responsive polymer materials. Nat Mater. 2010;9:101. - PubMed
-
- Zhang Q, Zhang Y, Wan Y, Carvalho W, Hu L, Serpe MJ. Stimuli-responsive polymers for sensing and reacting to environmental conditions. Prog Polym Sci. 2021;116: 101386.
LinkOut - more resources
Full Text Sources
