B cell CLL/lymphoma 10 promotes colorectal cancer cell proliferation and regulates cuproptosis sensitivity through the NF-κB signaling pathway
- PMID: 40937457
- PMCID: PMC12421401
- DOI: 10.3748/wjg.v31.i34.109825
B cell CLL/lymphoma 10 promotes colorectal cancer cell proliferation and regulates cuproptosis sensitivity through the NF-κB signaling pathway
Abstract
Background: Colorectal cancer (CRC) is a major global health burden. B cell CLL/lymphoma 10 (BCL10), a key component of the caspase recruitment domain protein-BCL10-mucosa-associated lymphoid tissue lymphoma paracaspase complexes, is upregulated in CRC and associated with poor patient prognosis, suggesting its potential role in CRC development and progression. Cuproptosis, a novel form of programmed cell death, has emerged as a promising therapeutic strategy for cancer.
Aim: To explore the role of BCL10 in regulating the sensitivity of CRC cells to cuproptosis.
Methods: A series of in vitro and in vivo experiments were conducted using CRC cell lines and CRC mouse models to evaluate the effects of BCL10 on CRC cell proliferation, migration, invasion, and sensitivity to copper-induced cell death. Mechanistic studies were performed to elucidate the underlying molecular pathways.
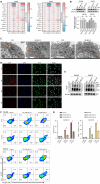
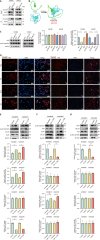
Results: BCL10 promoted CRC cell proliferation, migration, and invasion, while its knockdown had the opposite effects. BCL10 also influenced the sensitivity of CRC cells to cuproptosis, with BCL10 overexpression enhancing resistance and its knockdown increasing sensitivity. The mechanism involved BCL10 modulating the expression of DLAT, a key protein in the copper-induced cell death pathway, through activation of the nuclear factor kappa-B (NF-κB) signaling pathway.
Conclusion: BCL10 promotes CRC growth and regulates the sensitivity of CRC cells to cuproptosis by activating the NF-κB signaling pathway and modulating DLAT expression. These findings provide a molecular basis for developing BCL10-targeted therapies for CRC.
Keywords: B cell CLL/lymphoma 10; Cell death; Colorectal cancer; Cuproptosis; Nuclear factor kappa-B.
©The Author(s) 2025. Published by Baishideng Publishing Group Inc. All rights reserved.
Conflict of interest statement
Conflict-of-interest statement: All the authors report no relevant conflicts of interest for this article.
Figures





References
-
- Bray F, Laversanne M, Sung H, Ferlay J, Siegel RL, Soerjomataram I, Jemal A. Global cancer statistics 2022: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2024;74:229–263. - PubMed
-
- Sun L, Xing J, Zhou X, Song X, Gao S. Wnt/β-catenin signalling, epithelial-mesenchymal transition and crosslink signalling in colorectal cancer cells. Biomed Pharmacother. 2024;175:116685. - PubMed
-
- Li J, Li S, Xing X, Liu N, Lai S, Liao D, Li J. FTO-mediated ZNF687 accelerates tumor growth, metastasis, and angiogenesis in colorectal cancer through the Wnt/β-catenin pathway. Biotechnol Appl Biochem. 2024;71:245–255. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials

