Larval Ascariasis Triggers Unresolved Anemia, Persistent Inflammation, and Chronic Pulmonary Disease after Single and Reinfection in a Dose-Dependent Manner in Mice
- PMID: 40937693
- PMCID: PMC12519471
- DOI: 10.1021/acsinfecdis.5c00477
Larval Ascariasis Triggers Unresolved Anemia, Persistent Inflammation, and Chronic Pulmonary Disease after Single and Reinfection in a Dose-Dependent Manner in Mice
Abstract
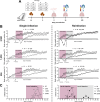
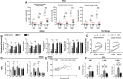
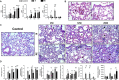
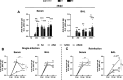
Although different studies have investigated the pathophysiological aspects of ascariasis using experimental models, the long-term effects following the peak of larval migration in the lungs remain poorly understood, especially in different doses of infection, such as those that mimic better the natural infection scenario. In this study, we evaluated the impact of different infection doses (250, 1250, and 2500 eggs) and amount of exposure (single vs reinfection) on the host's immune response over an extended period. Our findings demonstrate that even the lowest dose (250 eggs) can induce persistent, though less severe, pathological damages compared to higher doses. These include anemia resulting from alveolar hemorrhage and the development of chronic pulmonary disease. Notably, while lower doses elicit a milder immune response, clinical manifestations tend to appear later, indicating a delayed pathological impact. Importantly, inflammatory infiltrates and altered cytokine levels (including Th1/Th2/Th17) were still observed in the lungs more than 100 days postinfection, even after larval clearance. The humoral immune response against Ascaris suum remained active for at least 100 days postinfection, with the potential for longer persistence. Notably, even with lower doses, only two exposures were sufficient to trigger an immune pattern similar to that observed at higher doses. These findings highlight the need for further investigation into the chronic inflammatory effects of Ascaris infection, including its impact on distant organs and its potential contribution to the development of noncommunicable diseases and comorbidities. A better understanding of these mechanisms is essential for developing improved strategies to control ascariasis in endemic areas.
Keywords: ACO; chronic inflammation; comorbidities; larval ascariasis; lung disease; murine model.
Figures





References
-
- Holland C., Sepidarkish M., Deslyper G., Abdollahi A., Valizadeh S., Mollalo A., Mahjour S., Ghodsian S., Ardekani A., Behniafar H., Gasser R. B., Rostami A.. Global Prevalence of Ascaris Infection in Humans (2010–2021): A Systematic Review and Meta-Analysis. Infect. Dis. Poverty. 2022;11(1):113. doi: 10.1186/s40249-022-01038-z. - DOI - PMC - PubMed
-
- Gazzinelli-Guimarães P. H., Gazzinelli-Guimarães A. C., Silva F. N., Mati V. L. T., Dhom-Lemos L. D. C., Barbosa F. S., Passos L. S. A., Gaze S., Carneiro C. M., Bartholomeu D. C., Bueno L. L., Fujiwara R. T.. Parasitological and Immunological Aspects of Early Ascaris Spp. Infection in Mice. Int. J. Parasitol. 2013;43(9):697–706. doi: 10.1016/j.ijpara.2013.02.009. - DOI - PubMed
-
- Silva T. E. D., Barbosa F. S., Magalhães L. M. D., Gazzinelli-Guimarães P. H., Dos Santos A. C., Nogueira D. S., Resende N. M., Amorim C. C., Gazzinelli-Guimarães A. C., Viana A. G., Geiger S. M., Bartholomeu D. C., Fujiwara R. T., Bueno L. L.. Unraveling Ascaris Suum Experimental Infection in Humans. Microb. Infect. 2021;23(8):104836. doi: 10.1016/j.micinf.2021.104836. - DOI - PubMed
-
- Hotez P. J., Alvarado M., Basáñez M.-G., Bolliger I., Bourne R., Boussinesq M., Brooker S. J., Brown A. S., Buckle G., Budke C. M., Carabin H., Coffeng L. E., Fèvre E. M., Fürst T., Halasa Y. A., Jasrasaria R., Johns N. E., Keiser J., King C. H., Lozano R., Murdoch M. E., O’Hanlon S., Pion S. D. S., Pullan R. L., Ramaiah K. D., Roberts T., Shepard D. S., Smith J. L., Stolk W. A., Undurraga E. A., Utzinger J., Wang M., Murray C. J. L., Naghavi M.. The Global Burden of Disease Study 2010: Interpretation and Implications for the Neglected Tropical Diseases. PLoS Negl. Trop. Dis. 2014;8(7):e2865. doi: 10.1371/journal.pntd.0002865. - DOI - PMC - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

