Efficacy and Safety of GLP-1 and Dual GIP/GLP-1 Receptor Agonists in Idiopathic Intracranial Hypertension: A Systematic Review and Meta-Analysis
- PMID: 40937960
- PMCID: PMC12426903
- DOI: 10.1111/ene.70358
Efficacy and Safety of GLP-1 and Dual GIP/GLP-1 Receptor Agonists in Idiopathic Intracranial Hypertension: A Systematic Review and Meta-Analysis
Abstract
Background: Repurposing glucagon-like peptide-1 (GLP-1) and GIP/GLP-1 receptor agonists (RAs) for idiopathic intracranial hypertension (IIH) represents an attractive alternative to current treatments, supported by evidence of potent metabolic effects and reductions in cerebrospinal fluid secretion and intracranial pressure in vivo.
Methods: We evaluated the safety and efficacy of GLP-1 RAs and GIP/GLP-1 RAs in IIH. MEDLINE and Scopus databases were searched for randomized-controlled trials (RCT), non-randomized clinical trials, or registries in adults with IIH.
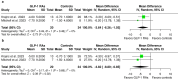
Results: Two clinical trials (one RCT and one non-randomized case-control) and two registries, comprising 1550 IIH patients (768 receiving GLP-1 or GIP/GLP-1 RAs) were included. Compared with standard-of-care, GLP-1 or GIP/GLP-1 RAs were associated with a significantly lower risk of papilledema (RR: 0.25; 95% CI: 0.15-0.43; p < 0.01) and visual disturbances or blindness (RR: 0.41; 95% CI: 0.18-0.92; p = 0.03), and a near-significant trend toward reduced headache risk (RR: 0.61; 95% CI: 0.34-1.07; p = 0.08). Additionally, GLP-1 RAs significantly reduced monthly headache days at 3 months (MD = -3.64; 95% CI: -6.26 to -1.03; p < 0.01) and at the end of follow-up (MD = -4.82; 95% CI: -8.80 to -0.85; p = 0.02). No association was detected between GLP-1 RAs and body mass index. No serious adverse events or treatment discontinuations were reported; mild gastrointestinal adverse events and nausea occurred in 88% (95% CI: 0.46-1.00) of GLP-1 RA-treated patients.
Conclusions: GLP-1 and dual GIP/GLP-1 RAs are associated with a significantly lower risk of papilledema and visual disturbances or blindness and a lower headache risk compared with standard-of-care. Additionally, GLP-1 RAs significantly reduce the monthly headache burden. Well-designed RCTs are needed to robustly evaluate the effects of GLP-1 and GIP/GLP-1 RAs in IIH, which likely extend beyond their weight-loss-inducing properties.
Trial registration: PROSPERO registration ID: CRD42025650082.
Keywords: GIP/GLP‐1; GLP‐1; exenatide; headache; intracranial hypertension; tirzepatide.
© 2025 The Author(s). European Journal of Neurology published by John Wiley & Sons Ltd on behalf of European Academy of Neurology.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures


References
-
- McCluskey G., Doherty‐Allan R., McCarron P., et al., “Meta‐Analysis and Systematic Review of Population‐Based Epidemiological Studies in Idiopathic Intracranial Hypertension,” European Journal of Neurology 25 (2018): 1218–1227. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

