Real-World Data on Inotuzumab Ozogamicin for Adult Patients With Relapsed/Refractory Acute Lymphoblastic Leukemia: A GRELAL-Chile Study
- PMID: 40938298
- PMCID: PMC12427359
- DOI: 10.1002/cam4.71230
Real-World Data on Inotuzumab Ozogamicin for Adult Patients With Relapsed/Refractory Acute Lymphoblastic Leukemia: A GRELAL-Chile Study
Abstract
Background: Inotuzumab Ozogamicin (InO) has shown efficacy in relapsed/refractory acute lymphoblastic leukemia (R/R ALL), but evidence from Latin America is scarce. We evaluated the outcomes of Chilean patients treated with InO in public and private health centers.
Methods: We retrospectively analyzed 35 patients with R/R ALL (median age 33 years; 54% male). Twenty percent had BCR::ABL-positive ALL, 78% expressed CD22, and 91% expressed CD19. Response rates, measurable residual disease (MRD), survival outcomes, and treatment-related toxicities were assessed. Multivariate analyses explored prognostic factors.
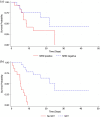
Results: Complete remission or remission with incomplete recovery (CR/CRi) was achieved in 74% of patients. Among those evaluated, 82% reached MRD < 0.01%. Patients undergoing allogeneic hematopoietic stem cell transplantation (Allo-HSCT) after InO had superior overall survival (OS) compared with those who did not (24.2 vs. 5.2 months). Median progression-free survival (PFS) was 6.9 months and median OS was 8.8 months. Sinusoidal obstruction syndrome occurred in 14% of patients but was generally mild. Multivariate analysis identified comorbidities and high blast counts as adverse prognostic factors, whereas MRD negativity and subsequent Allo-HSCT were associated with improved outcomes.
Conclusions: InO demonstrated high remission and MRD negativity rates in Chilean patients with R/R ALL, with OS and PFS comparable to existing research. Although SOS incidence was higher, it was generally mild. Achieving MRD negativity and proceeding to Allo-HSCT provided the greatest survival benefit. Study limitations include short follow-up and limited data.
Keywords: Inotuzumab Ozogamicin; acute lymphoblastic leukemia; sinusoidal obstruction syndrome.
© 2025 The Author(s). Cancer Medicine published by John Wiley & Sons Ltd.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures


References
-
- Kantarjian H., Thomas D., O'Brien S., et al., “Long‐Term Follow‐Up Results of Hyperfractionated Cyclophosphamide, Vincristine, Doxorubicin, and Dexamethasone (Hyper‐CVAD), a Dose‐Intensive Regimen, in Adult Acute Lymphocytic Leukemia,” Cancer 101 (2004): 2788–2801, 10.1002/cncr.20668. - DOI - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

