Prospective characterization of germline variants in patients with gliomas and glioneuronal tumors
- PMID: 40938445
- PMCID: PMC12432092
- DOI: 10.1007/s00401-025-02935-x
Prospective characterization of germline variants in patients with gliomas and glioneuronal tumors
Abstract
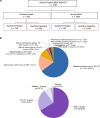
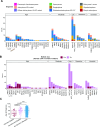
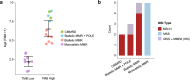
Several tumor predisposition syndromes have been linked to the development of gliomas and glioneuronal tumors (glioma/GNT). For many pathogenic germline variants, the prevalence and clinical significance remain unclear. Germline variants and copy-number variants affecting 76-90 well-established cancer predisposing genes were identified in 2,187 patients with gliomas/GNT, who underwent prospective sequencing of their tumor and a matched normal sample. A germline pathogenic or likely pathogenic (P/LP) mutation was identified in 11% (250/2187, 95% CI 10.1-12.8%). Affected high- and moderate-penetrance genes included BRCA2 (n = 11; 0.5%), TP53 (n = 8; 0.4%), NF1 (n = 8; 0.4%), CHEK2 (n = 21, 0.9% excluding common variant I157T), and the mismatch repair (MMR) genes (n = 22, 1.0%). Biallelic inactivation was identified in 8/8 tumors with a germline NF1 mutation, 7/8 tumors with a germline TP53 alteration, and 10/19 tumors with a heterozygous germline MMR defect. Gliomas/GNT with biallelic inactivation of an MMR gene were characterized by hypermutation, microsatellite instability, and a distinct clinical phenotype. Assessment of zygosity identifies biallelic inactivation of DNA double-strand break repair alterations in a minority of tumors, including BRCA2-deficient gliomas with increased genomic scarring attributable to homologous recombination deficiency, and refutes the contribution of the most common P/LP germline variants. Irrespective of gene, tumors with biallelic inactivation were diagnosed at a younger age than tumors without a germline variant (p = 3.5 × 10-6) and tumors with a monoallelic alteration (p = 0.00014). In conclusion, germline sequencing identifies a P/LP variant in a high proportion of patients with glioma/GNT. Biallelic inactivation was common in younger patients with germline variants and patients with neurofibromatosis type 1/Li-Fraumeni, but was only present in half of the patients with Lynch syndrome.
Keywords: Cancer predisposition syndrome; Germline sequencing; Li-Fraumeni syndrome; Lynch syndrome; Neurofibromatosis type 1.
© 2025. The Author(s).
Conflict of interest statement
Declarations. Conflict of Interest: R.J. Young reports equity in Agios, and consulting fees from Guerbet, ICON plc, NordicNeuroLab, Olea Medical, RadMD, Servier, and Turing Medical, unrelated to the present work. N.S. Moss has consulted/received speaking fees from AstraZeneca, Daiichi Sankyo and Varian, and received research funding (to institution) from AZ, Amgen and GT. A. Boire is an inventor on the following patents: 62/258,044, 10/413,522, and 63/052,139. C. Grommes reports honoraria from Scripps Health; consulting or advisory roles from BTG, Kite/Gilead, Ono Pharmaceutical, Roche, Curis; speakers’ bureau from Ono Pharmaceutical; travel, accommodations, and expenses from Ono Pharmaceutical, Bayer, Bristol Myers Squibb, Pharmacyclics, Celgene, unrelated to the present work. B.D. Santomasso has received consulting honoraria from Bristol-Myers Squibb, Johnson & Johnson, Janssen, and Gilead and serves on the scientific advisory board of In8bio, all unrelated to the present work. E.L. Diamond discloses unpaid editorial support from Pfizer Inc, unrelated to the present work. T.J. Kaley reports other support from Servier, unrelated to the present work. I.K. Mellinghoff reports researcher fees from Servier, Erasca, Abbvie, Roche, and Global Coalition for Adaptive Research, speaker fees from Baptist Health Care, grants from Indiana University School of Medicine, and advisory board fees from Tango Therapeutics and Pathos Inc, all unrelated to the present work. M. Berger reports personal fees from AstraZeneca and Paige.AI, research support from Boundless Bio, and intellectual property rights from SOPHiA Genetics. Z.K. Stadler has intellectual property rights in SOPHiA Genetics and serves as an Associate Editor for JCO Precision Oncology and as a Section Editor for UpToDate. Z.K. Stadler's immediate family member serves on the Board of Directors for Adverum Biotechnologies, is Co-Founder, CMO and President for Blue Gen Therapeutics Foundation, and serves as a consultant in Ophthalmology for Apellis, Gyroscope, Nanoscope, Novartis, Outlook Therapeutics, and Regeneron unrelated to the present work. A.L. Lin reports funding from Bristol Myers Squibb, unrelated to the present work. All remaining authors have declared no conflicts of interest. Ethical approval: Patients in this study were enrolled on a sequencing protocol (NCT01775072) that was approved by the Institutional Review Board of Memorial Sloan Kettering Cancer Center and conducted in accordance with International Ethical Guidelines for Biomedical Research Involving Human Subjects, Good Clinical Practice guidelines, the Declaration of Helsinki, as well as federal laws, after providing written informed consent.
Figures
References
-
- Attard TM, Giglio P, Koppula S, Snyder C, Lynch HT (2007) Brain tumors in individuals with familial adenomatous polyposis: a cancer registry experience and pooled case report analysis. Cancer 109:761–766. 10.1002/cncr.22475 - PubMed
-
- Cheng DT, Mitchell TN, Zehir A, Shah RH, Benayed R, Syed A et al (2015) Memorial Sloan Kettering-Integrated Mutation Profiling of Actionable Cancer Targets (MSK-IMPACT): a hybridization capture-based next-generation sequencing clinical assay for solid tumor molecular oncology. J Mol Diagn 17:251–264. 10.1016/j.jmoldx.2014.12.006 - PMC - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

