M2 Macrophages are Major Mediators of Germline Risk of Endometriosis and Explain Pleiotropy With Comorbid Traits
- PMID: 40938538
- PMCID: PMC12591210
- DOI: 10.1002/advs.202415285
M2 Macrophages are Major Mediators of Germline Risk of Endometriosis and Explain Pleiotropy With Comorbid Traits
Abstract
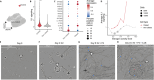
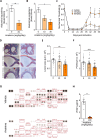
Endometriosis is a common gynecologic condition that causes chronic, life-altering symptoms including pain and infertility. There is an urgent need for new non-hormonal targeted therapeutics to treat endometriosis, but until very recently, the cellular and molecular signatures of endometriotic lesions are undefined, hindering the development of clinical advances. Integrating inherited risk data from analyses of >45 0000 individuals with ≈35 0000 single-cell transcriptomes from 21 patients, M2-macrophages as candidate drivers of disease susceptibility are uncovered, and nominating IL1 signaling as a central hub impacted by germline genetic variation associated with endometriosis risk. Extensive functional follow-up confirmed these associations and revealed a pleiotropic role for this pathway in endometriosis. Population-scale expression quantitative trait locus analysis demonstrates that genetic variation controlling IL1A expression is associated with endometriosis risk variants. Manipulation of IL1 signaling in state-of-the-art in vitro decidualized endometrial organoids impacts epithelial differentiation, and in an in vivo endometriosis model, treatment with anakinra (an interleukin-1 receptor antagonist) results in a significant, dose-dependent reduction in spontaneous and evoked pain and dampened pro-angiogenic signaling. Together, these studies highlight non-diagnostic cell types as central to endometriosis susceptibility and support IL1 signaling as an important actionable pathway for this disease.
Keywords: GWAS; IL1A; IL1B; M2 macrophages; anakinra; angiogenesis; endometriosis; inflammation; organoids; single cell transcriptomics.
© 2025 The Author(s). Advanced Science published by Wiley‐VCH GmbH.
Conflict of interest statement
The authors declare no conflict of interest.
Figures




Update of
-
M2 Macrophages are Major Mediators of Germline Risk of Endometriosis and Explain Pleiotropy with Comorbid Traits.bioRxiv [Preprint]. 2024 Nov 22:2024.11.21.624726. doi: 10.1101/2024.11.21.624726. bioRxiv. 2024. Update in: Adv Sci (Weinh). 2025 Nov;12(41):e15285. doi: 10.1002/advs.202415285. PMID: 39605445 Free PMC article. Updated. Preprint.
References
-
- Shim J. Y., Laufer M. R., Grimstad F. W., J. Pediatr. Adolesc. Gynecol. 2020, 33, 524. - PubMed
-
- Pearce C. L., Templeman C., Rossing M. A., Lee A., Near A. M., Webb P. M., Nagle C. M., Doherty J. A., Cushing‐Haugen K. L., Wicklund K. G., Chang‐Claude J., Hein R., Lurie G., Wilkens L. R., Carney M. E., Goodman M. T., Moysich K., Kjaer S. K., Hogdall E., Jensen A., Goode E. L., Fridley B. L., Larson M. C., Schildkraut J. M., Palmieri R. T., Cramer D. W., Terry K. L., Vitonis A. F., Titus L. J., Ziogas A., Lancet Oncol. 2012, 13, 385. - PMC - PubMed
-
- Kvaskoff M., Mahamat‐Saleh Y., Farland L. V., Shigesi N., Terry K. L., Harris H. R., Roman H., Becker C. M., As‐Sanie S., Zondervan K. T., Horne A. W., Missmer S. A., Hum. Reprod. Update 2021, 27, 393. - PubMed
-
- Zhang T., De Carolis C., Man G. C. W., Wang C. C., Autoimmun. Rev. 2018, 17, 945. - PubMed
MeSH terms
Substances
Grants and funding
- The Cedars-Sinai grant supportc
- GNT1177194/National Health and Medical Research Council of Australia Investigator Grants
- R01HD113693/Eunice Kennedy Shriver National Institute of Child Health and Human Development
- GNT1173882/National Health and Medical Research Council of Australia Investigator Grants
- W81XWH1910364/U.S. Department of Defense
- R01 HD113693/HD/NICHD NIH HHS/United States
- R01 HD114855/HD/NICHD NIH HHS/United States
- UL1TR001881/TR/NCATS NIH HHS/United States
- R01HD114855/Eunice Kennedy Shriver National Institute of Child Health and Human Development
- 134005/Cedars-Sinai Medical Center
- The JW and Alice Marriott Foundation
- R01 HG013258/HG/NHGRI NIH HHS/United States
- R01HG013258/Eunice Kennedy Shriver National Institute of Child Health and Human Development
- UL1 TR001881/TR/NCATS NIH HHS/United States
- The Marriott Daughters foundation
LinkOut - more resources
Full Text Sources
Medical
