Phase 2 trial of personal dendritic cell vaccines in newly diagnosed glioblastoma: 3-year follow-up and correlations with survival
- PMID: 40938661
- PMCID: PMC12439551
- DOI: 10.1080/21645515.2025.2556591
Phase 2 trial of personal dendritic cell vaccines in newly diagnosed glioblastoma: 3-year follow-up and correlations with survival
Abstract
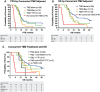
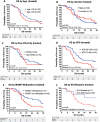
Survival of glioblastoma (GBM) patients is unsatisfactory. Adding patient-specific vaccines to standard therapy may improve outcomes. Autologous dendritic cells (DC) loaded with autologous tumor antigens (ATA) from short-term cell lines established from autologous tumor are a promising therapeutic vaccine strategy. DC-ATA vaccines were tested in a single-arm phase 2 trial in newly diagnosed GBM patients. Tumor tissue was collected during surgical resection. DC were differentiated from peripheral blood monocytes. Intent-to-treat enrollment took place before standard concurrent radiation therapy and temozolomide (RT/TMZ). DC-ATA was manufactured during RT/TMZ, then injected subcutaneously over 6 months concurrently with adjuvant TMZ. DC-ATA was reliably manufactured; treatment was well tolerated. Fifty-seven patients started vaccine therapy; 69% received all eight vaccine doses. The 10.7-month median progression-free survival (PFS) is 57% greater than the average of medians from standard treatment arms in six randomized trials that also enrolled patients before RT/TMZ. Disease control and overall survival (OS) dropped precipitously once DC-ATA was completed. Survival curves stratified by prognostic variables did not separate until after DC-ATA was discontinued. Multivariate analysis identified receipt of all eight planned vaccine injections, MGMT promoter methylation, concurrent dexamethasone dose ≤2 mg, and receipt of six or more TMZ cycles as independent variables. Common features among the seven patients who were progression-free after 3 years were eight vaccine injections, ≤2 mg dexamethasone, age <60 years, and adjuvant TMZ given without concurrent bevacizumab. PFS was encouraging, and the data suggest that OS may be increased by extending vaccine treatment.
Keywords: Glioblastoma; autologous tumor antigens; dendritic cell vaccine.
Conflict of interest statement
RM Robles, K Godding, HS Keirstead, GI Nistor, and RO Dillman are employees of AIVITA Biomedical, Inc.
Figures





References
-
- NCCN Guidelines Version 5 . Central nervous system cancers. Adult glioma: glioblastoma, GLIO-10. 2024. 2025 Mar 18.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
