Metabolism-driven posttranslational modifications and immune regulation: Emerging targets for immunotherapy
- PMID: 40938975
- PMCID: PMC12428940
- DOI: 10.1126/sciadv.adx6489
Metabolism-driven posttranslational modifications and immune regulation: Emerging targets for immunotherapy
Abstract
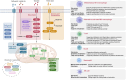
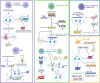
The interplay between cellular metabolism and immune regulation is central to immune function and disease progression, revealing notable therapeutic opportunities. Upon activation, immune cells undergo metabolic reprogramming to meet heightened demands for energy and biosynthesis, reshaping regulatory networks across epigenomic, transcriptomic, and proteomic layers. Metabolite-derived posttranslational modifications (PTMs) serve as pivotal mechanisms integrating metabolic intermediates with immune signaling pathways. Beyond classical acetylation, diverse nonacetyl PTMs-including lactylation, succinylation, malonylation, palmitoylation, and myristoylation-modify histone and nonhistone proteins, regulating gene expression, protein stability, subcellular localization, enzymatic activity, and protein-protein interactions. Advances in mass spectrometry and bioinformatics now enable precise characterization of these PTMs, uncovering their broad roles in immune regulation. This review summarizes current progress in immunometabolism and explores future directions such as mechanistic studies, combination strategies, and clinical applications. Metabolite-driven PTMs critically connect metabolism to immune regulation, suggesting promising therapeutic approaches for cancer, autoimmune disorders, and inflammatory diseases.
Figures


References
-
- Ryan D. G., O’Neill L. A. J., Krebs cycle reborn in macrophage immunometabolism. Annu. Rev. Immunol. 38, 289–313 (2020). - PubMed
-
- Palsson-McDermott E. M., Curtis A. M., Goel G., Lauterbach M. A., Sheedy F. J., Gleeson L. E., van den Bosch M. W., Quinn S. R., Domingo-Fernandez R., Johnston D. G., Jiang J. K., Israelsen W. J., Keane J., Thomas C., Clish C., Vander Heiden M., Xavier R. J., O’Neill L., Pyruvate kinase M2 regulates Hif-1α activity and IL-1β induction and is a critical determinant of the Warburg effect in LPS-activated macrophages. Cell Metab. 21, 347–347 (2015). - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Miscellaneous

