Elucidating the mechanism by which HIV-1 nucleocapsid mutations confer resistance to integrase strand transfer inhibitors
- PMID: 40938996
- PMCID: PMC12429003
- DOI: 10.1126/sciadv.adz8980
Elucidating the mechanism by which HIV-1 nucleocapsid mutations confer resistance to integrase strand transfer inhibitors
Abstract
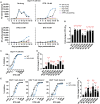
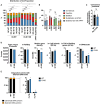
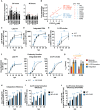
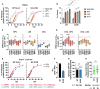
Persons with HIV (PWH) receiving integrase (IN) strand transfer inhibitors (INSTIs) have been reported to experience virologic failure (VF) in the absence of resistance mutations in IN. We previously reported that mutations in the viral nucleocapsid (NC) are selected in the presence of the INSTI dolutegravir (DTG). Here, we show that these NC mutations accelerate the kinetics of viral DNA integration, suggesting that they limit the window of time available for INSTIs to block viral DNA integration. We find that in primary peripheral blood mononuclear cells, HIV-1 acquires mutations in the viral envelope glycoprotein, NC, and occasionally IN during selection for INSTI resistance. Notably, the selected NC and IN mutations act in concert to reduce the susceptibility of the virus to INSTIs. These results provide insights into the mechanism by which HIV-1 escapes the inhibitory activity of INSTIs and underscore the importance of genotypic analysis outside IN in PWH experiencing VF on INSTI-containing drug regimens.
Figures

Update of
-
Elucidating the Mechanism by Which HIV-1 Nucleocapsid Mutations Confer Resistance to Integrase Strand Transfer Inhibitors.bioRxiv [Preprint]. 2025 May 18:2025.05.17.654662. doi: 10.1101/2025.05.17.654662. bioRxiv. 2025. Update in: Sci Adv. 2025 Sep 12;11(37):eadz8980. doi: 10.1126/sciadv.adz8980. PMID: 41030995 Free PMC article. Updated. Preprint.
References
-
- Gandhi R. T., Bedimo R., Hoy J. F., Landovitz R. J., Smith D. M., Eaton E. F., Lehmann C., Springer S. A., Sax P. E., Thompson M. A., Benson C. A., Buchbinder S. P., Del Rio C., Eron J. J. Jr., Gunthard H. F., Molina J. M., Jacobsen D. M., Saag M. S., Antiretroviral drugs for treatment and prevention of HIV infection in adults: 2022 Recommendations of the international antiviral society-USA panel. JAMA 329, 63–84 (2023). - PubMed
-
- World Health Organization, HIV drug resistance: Brief report 2024, (2024); https://www.who.int/publications/i/item/9789240086319.
-
- Chen H., Wei S. Q., Engelman A., Multiple integrase functions are required to form the native structure of the human immunodeficiency virus type I intasome. J. Biol. Chem. 274, 17358–17364 (1999). - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

