The impact of Asic2 deletion on metabolic homeostasis in mice
- PMID: 40939131
- PMCID: PMC12431597
- DOI: 10.14814/phy2.70514
The impact of Asic2 deletion on metabolic homeostasis in mice
Abstract
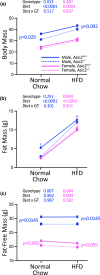
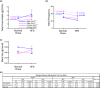
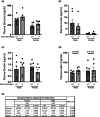
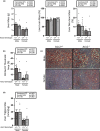
Acid-Sensing Ion Channel 2 (Asic2) is expressed in many brain regions, including the hypothalamus, the homeostatic center for hunger-satiety-energy expenditure. We used the HypoMap reference atlas of the mouse hypothalamus to localize Asic2 message in the specific hypothalamic regions and found that Asic2 is the predominant degenerin subunit expressed in mouse hypothalamic neurons in the ventromedial, lateral, and arcuate regions and is expressed in pro-opiomelanocortin (Pomc), agouti-related peptide (Agrp), as well as leptin (Lepr) and insulin receptors (Insr) positive neurons. Thus, we investigated if Asic2 plays an important role in body weight control and energy expenditure before and after high-fat diet (HFD). Asic2-/- male mice had lower lean body mass. On normal chow, Asic2-/- mice consumed more food, displayed elevated total energy expenditure, and a higher respiratory exchange ratio compared to WT mice. On the HFD, Asic2-/- female mice had greater motor activity and resting and total energy expenditure. We found no effect of genotype on plasma glucose, insulin, or ghrelin levels; however, leptin was elevated in male Asic2-/- mice following the HFD. Our findings suggest that Asic2 plays an important role in metabolic homeostasis, possibly through regulation of central pathways involved in energy balance.
Keywords: degenerin; diet induced obesity; energy balance.
© 2025 The Author(s). Physiological Reports published by Wiley Periodicals LLC on behalf of The Physiological Society and the American Physiological Society.
Figures




References
-
- Badmus, O. O. , Kipp, Z. A. , Bates, E. A. , da Silva, A. A. , Taylor, L. C. , Martinez, G. J. , Lee, W. H. , Creeden, J. F. , Hinds, T. D., Jr. , & Stec, D. E. (2023). Loss of hepatic PPARalpha in mice causes hypertension and cardiovascular disease. American Journal of Physiology. Regulatory, Integrative and Comparative Physiology, 325(1), R81–R95. - PMC - PubMed
-
- Barbaro, N. R. , Foss, J. D. , Kryshtal, D. O. , Tsyba, N. , Kumaresan, S. , Xiao, L. , Mernaugh, R. L. , Itani, H. A. , Loperena, R. , Chen, W. , Dikalov, S. , Titze, J. M. , Knollmann, B. C. , Harrison, D. G. , & Kirabo, A. (2017). Dendritic cell Amiloride‐sensitive channels mediate sodium‐induced inflammation and hypertension. Cell Reports, 21(4), 1009–1020. - PMC - PubMed
-
- Bassilana, F. , Champigny, G. , Waldmann, R. , de Weille, J. R. , Heurteaux, C. , & Lazdunski, M. (1997). The acid‐sensitive ionic channel subunit ASIC and the mammalian degenerin MDEG form a heteromultimeric H+‐gated Na+ channel with novel properties. The Journal of Biological Chemistry, 272(46), 28819–28822. - PubMed
MeSH terms
Substances
Grants and funding
- R01 DK124327/DK/NIDDK NIH HHS/United States
- P20GM144041/HHS | NIH | National Institute of General Medical Sciences (NIGMS)
- 5T32HL105324/HHS | NIH | National Heart, Lung, and Blood Institute (NHLBI)
- P20 GM104357/GM/NIGMS NIH HHS/United States
- R01HL163076/HHS | NIH | National Heart, Lung, and Blood Institute (NHLBI)
- R01DK137167/HHS | NIH | National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK)
- P20GM104357/HHS | NIH | National Institute of General Medical Sciences (NIGMS)
- R01DK124327/HHS | NIH | National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK)
- R01 HL163076/HL/NHLBI NIH HHS/United States
- R01 DK137167/DK/NIDDK NIH HHS/United States
- P20 GM144041/GM/NIGMS NIH HHS/United States
- T32 HL105324/HL/NHLBI NIH HHS/United States
LinkOut - more resources
Full Text Sources
Miscellaneous

