Randomised controlled trial to measure effectiveness and cost-effectiveness of a digital social intervention promoted by primary care clinicians to adults with asthma to improve asthma control: protocol
- PMID: 40940055
- PMCID: PMC12434742
- DOI: 10.1136/bmjopen-2025-104367
Randomised controlled trial to measure effectiveness and cost-effectiveness of a digital social intervention promoted by primary care clinicians to adults with asthma to improve asthma control: protocol
Abstract
Introduction: In the UK, approximately 5.4 million adults live with asthma, of whom one in five have an uncontrolled form. Uncontrolled asthma reduces quality of life and increases healthcare use. Engaging with peers through online health communities (OHCs) can empower patients to self-manage their long-term condition. While OHCs have been in existence for several years and growing numbers of patients access them, the role of primary care in signposting patients to them has been minimal and ad hoc. We have co-developed with patients and healthcare professionals (HCPs) an intervention for adult patients with asthma, consisting of an appointment with a primary care HCP to introduce online peer support and sign patients up to an established asthma OHC, followed by OHC engagement. Feasibility work found the intervention acceptable to patients and HCPs. This protocol outlines our plan to test the intervention's effectiveness and cost-effectiveness.
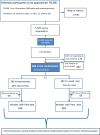
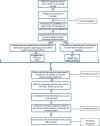
Methods and analysis: An individual randomised controlled trial will be carried out. Eligible participants will be recruited via an online survey sent to adult patients on the asthma register in 50-70 general practices in several UK locations. Participants will be invited to attend a one-off, face-to-face appointment with a primary care HCP, during which they will be individually randomised to the intervention or usual care. An asthma control test (primary outcome) and other measures of clinical effectiveness will be collected at baseline and every 3 months over a 12-month follow-up period. Descriptive and inferential statistics will be used to compare outcome measures between study arms. Cost-effectiveness assessment of the intervention compared with current standard of asthma management in primary care will be reported. A sample of patients and HCPs will be interviewed at study exit and the data analysed thematically.
Ethics and dissemination: The study was approved by a National Health Service Research Ethics Committee (reference: 25/NE/0006). Written consent will be obtained from all participants. Findings will be disseminated through various means, including sharing with general practices, conference presentations and peer-reviewed publications.
Trial registration number: NCT06849245.
Keywords: asthma; primary care; protocols & guidelines; randomized controlled trial; self care; social media.
© Author(s) (or their employer(s)) 2025. Re-use permitted under CC BY. Published by BMJ Group.
Conflict of interest statement
Competing interests: None declared.
Figures
References
-
- Asthma + Lung UK. Welcome to Asthma + Lung UK. 2025. [10-Mar-2025]. https://www.asthmaandlung.org.uk/ Available. Accessed.
-
- Types of asthma Asthma + Lung UK. 2024. [09-Jan-2025]. https://www.asthmaandlung.org.uk/conditions/asthma/types-asthma#severe-a... Available. Accessed.
-
- NHS RightCare RightCare asthma scenario. 2023. [09-Jan-2025]. https://www.england.nhs.uk/wp-content/uploads/2023/02/Rightcare-Asthma-S... Available. Accessed.
-
- Asthma + Lung UK Lung conditions kill more people in the UK than anywhere in Western Europe. 2022. [09-Jan-2025]. https://www.asthmaandlung.org.uk/media/press-releases/lung-conditions-ki... Available. Accessed.
Publication types
MeSH terms
Associated data
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous