Dietary supplementation of arachidonic acid promotes humoral immunity
- PMID: 40940568
- PMCID: PMC12603062
- DOI: 10.1038/s44321-025-00310-7
Dietary supplementation of arachidonic acid promotes humoral immunity
Abstract
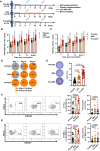
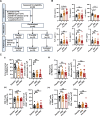
Vaccination offers the most effective protection against contagious infectious diseases primarily by inducing humoral immunity. Vaccination efficacy is influenced by various factors. We report that dietary administration of arachidonic acid (ARA) significantly boosts rabies vaccine-induced production of neutralizing antibodies and protection against lethal rabies virus (RABV) infection in mice. In human volunteers, oral supplementation of ARA accelerates the expression of neutralizing antibodies to the levels sufficient for protection against RABV as early as one week after primary immunization. Mechanistically, ARA is enriched in lymph nodes and metabolized into immune modulators there. One of the ARA metabolites, prostaglandin I2 (PGI2), via the cyclic adenosine monophosphate (cAMP)-protein kinase A (PKA) axis, upregulates the expression of costimulatory molecule CD86, and activates activation-induced cytidine deaminase (AID) in B cells. These results suggest that ARA can be a potent dietary adjuvant to foster germinal center (GC) B cell response and humoral immunity.
Keywords: Arachidonic Acid; Germinal Center Response; Humoral Immunity.
© 2025. The Author(s).
Conflict of interest statement
Disclosure and competing interests statement. The authors declare no competing interests.
Figures







References
-
- Barbier AJ, Jiang AY, Zhang P, Wooster R, Anderson DG (2022) The clinical progress of mRNA vaccines and immunotherapies. Nat Biotechnol 40:840–854 - PubMed
-
- Barreto V, Reina-San-Martin B, Ramiro AR, McBride KM, Nussenzweig MC (2003) C-terminal deletion of AID uncouples class switch recombination from somatic hypermutation and gene conversion. Mol Cell 12:501–508 - PubMed
-
- Borriello F, Sethna MP, Boyd SD, Schweitzer AN, Tivol EA, Jacoby D, Strom TB, Simpson EM, Freeman GJ, Sharpe AH (1997) B7-1 and B7-2 have overlapping, critical roles in immunoglobulin class switching and germinal center formation. Immunity 6:303–313 - PubMed
MeSH terms
Substances
Grants and funding
- 32188101/MOST | National Natural Science Foundation of China (NSFC)
- 82271872/MOST | National Natural Science Foundation of China (NSFC)
- 82341046/MOST | National Natural Science Foundation of China (NSFC)
- B2404002/Shenzhen Medical Research Fund
- 202502AU100001/Yunnan Major Scientific and Technological Projects
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

