Increased risk of infections in smoldering multiple myeloma: results from the screened iStopMM study
- PMID: 40940581
- PMCID: PMC12634437
- DOI: 10.1038/s41375-025-02762-9
Increased risk of infections in smoldering multiple myeloma: results from the screened iStopMM study
Abstract
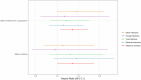
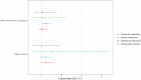
Infections are a major cause of morbidity and mortality in multiple myeloma (MM). While increased infection risk has been shown in monoclonal gammopathy of undetermined significance (MGUS), data are limited for smoldering multiple myeloma (SMM). We used data from the iStopMM study, which screened 75,422 Icelandic individuals aged ≥40 years for MM precursors. Individuals diagnosed with SMM were matched by age and sex with MGUS-free comparators (1:5 ratio) and with individuals with MGUS (1:1 ratio). Infection outcomes were derived from nationwide registries of ICD-10 diagnostic codes and antimicrobial prescriptions. Cox proportional hazards models estimated infection risk, adjusted for immunoparesis. 188 SMM individuals were matched to 188 MGUS individuals and 162 SMM individuals were matched to 810 comparators. Individuals with SMM had significantly more infections (HR 1.36, 95% CI 1.07-1.73) and antibacterial prescriptions (HR 1.24, 95% CI 1.01-1.52) than the comparators. Compared to MGUS, individuals with SMM also had more infections (HR 1.37, 95% CI 1.00-1.87). Adjusting for immunoparesis attenuated the associations, suggesting it may partially mediate infection risk. This first screened cohort of SMM shows a significantly increased infection risk, compared to both MGUS and to individuals without MM precursors, suggesting an underrecognized infection burden in SMM.
© 2025. The Author(s).
Conflict of interest statement
Competing interests: LSA has participated in a conference supported by Alexion. SR: Honoraria for scientific talks from Johnson & Johnson and Siemens Healthineers. SH: Current employment at The Binding Site part of Thermo Fisher Scientific. OL: Research funding from NCI/NIH, FDA, LLS, Rising Tide Foundation, MMRF, IMF, Paula and Rodger Riney Foundation, Tow Foundation, Myeloma Solutions Fund, Perelman Family Foundation, Amgen, Celgene, Janssen, Takeda, Glenmark, Seattle Genetics, Karyopharm; Honoraria for scientific talks/participated in advisory boards for: Adaptive, Amgen, Binding Site, BMS, Celgene, Cellectis, Glenmark, Janssen, Juno, Pfizer; Independent Data Monitoring Committees for: Takeda, Merck, Janssen. SYK: Research funding from Amgen and Celgene; Independent Data Monitoring Committee for Jansen. ST: Honoraria for scientific talks/participated in advisory boards for Sanofi, Abbvie and the Binding Site, and travel grant from Amgen. ISS: Advisory board for Sanofi. The remaining authors declare no competing interests. Ethics approval and consent to participate: The iStopMM study protocol was approved by the Icelandic National Bioethics Committee (Number 16-022, date: 2016-04-06) with approval from the Icelandic Data Protection Agency. Access to national healthcare registries has been approved by the Icelandic Directorate of Health and the Icelandic Cancer Society. Informed consent was obtained from all participants. All methods were performed in compliance with all relevant guidelines and regulations for use of Icelandic data.
Figures
References
-
- Rajkumar SV, Dimopoulos MA, Palumbo A, Blade J, Merlini G, Mateos MV, et al. International Myeloma Working Group updated criteria for the diagnosis of multiple myeloma. Lancet Oncol. 2014. 15:e538–48. - PubMed
-
- Nucci M, Anaissie E. Infections in patients with multiple myeloma. Semin Hematol. 2009;46:277–88. - PubMed
MeSH terms
LinkOut - more resources
Full Text Sources
Medical

