Targeting Diabetic Retinopathy with Human iPSC-Derived Vascular Reparative Cells in a Type 2 Diabetes Model
- PMID: 40940763
- PMCID: PMC12428381
- DOI: 10.3390/cells14171352
Targeting Diabetic Retinopathy with Human iPSC-Derived Vascular Reparative Cells in a Type 2 Diabetes Model
Abstract
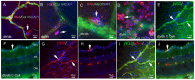
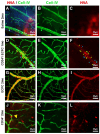
Purpose: To investigate the therapeutic potential of inducible pluripotent stem cell (hiPSC)-based vascular repair, we evaluated two vascular reparative cell populations, CD34+ cells derived from hiPSC (hiPSC-CD34+) and endothelial colony forming cells (ECFCs) derived from hiPSC (iPS-ECFCs), alone and in combination, in a type 2 diabetic (db/db) mouse model of DR. Methods: hiPSC-CD34+ cells (1 × 104) or iPSC- ECFCs (1 × 105) alone or in combination (1.1 × 105) were injected into the vitreous of immunosuppressed db/db mice with six months of established diabetes. One month post-injection, mice underwent electroretinography (ERG) and optical coherence tomography (OCT) to evaluate functional and structural retinal recovery with iPSC administration. Immunohistochemistry (IHC) was used to assess recruitment and incorporation of cells into the retinal vasculature. Retinas from the experimental groups were analyzed using Functional Proteomics via Reverse Phase Protein Array (RPPA). Results: Functional assessment via ERG demonstrated significant improvements in retinal response in the diabetic cohorts treated with either hiPSC-derived CD34+ cells or hiPSC-ECFCs. Retinal thickness, assessed by OCT, was restored to near-nondiabetic levels in mice treated with hiPSC-CD34+ cells alone and the combination group, whereas hiPSC-ECFCs alone did not significantly affect retinal thickness. One month following intravitreal injection, hiPSC-CD34+ cells were localized to perivascular regions, whereas hiPSC-ECFCs were observed to integrate directly into the retinal vasculature. RPPA analysis revealed interaction-significant changes, and this was interpreted as a combination-specific, non-additive host responses (m6A, PI3K-AKT-mTOR, glycolysis, endothelial junction pathways). Conclusions: The studies support that injection of hiPSC-CD34+ cells and hiPSC-ECFCs, both individually and in combination, showed benefit; however, iPSC combination-specific effects were identified by measurement of retinal thickness and by RPPA.
Keywords: CD34 cells; KNA cells; diabetic retinopathy; endothelial colony forming cells; inducible pluripotent stem cells; vascular repair.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures



References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

