Dinutuximab Beta Versus Naxitamab in the Treatment of Relapsed/Refractory Neuroblastoma in Patients with Stable Disease, Minor Response or Partial Response and Disease in Bone or Bone Marrow: Systematic Review and Matching-Adjusted Indirect Comparison
- PMID: 40940820
- PMCID: PMC12427561
- DOI: 10.3390/cancers17172723
Dinutuximab Beta Versus Naxitamab in the Treatment of Relapsed/Refractory Neuroblastoma in Patients with Stable Disease, Minor Response or Partial Response and Disease in Bone or Bone Marrow: Systematic Review and Matching-Adjusted Indirect Comparison
Abstract
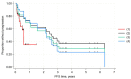
Objective: Dinutuximab beta (DB) and naxitamab (NAXI) with GM-CSF are used for maintenance treatment of relapsed/refractory neuroblastoma. The objective of this study was to systematically assess comparative efficacy of the two therapies within their designated indications in accordance with established clinical guidelines. Methods: Relevant evidence was identified in systematic literature review. Individual patient data (IPD) from prospective clinical trials of DB were assessed and data on patients with disease in bone or bone marrow, as assessed in MRI, CT, mIBG or biopsy, with incomplete response to previous therapy were included. Patients with complete response, progressive disease and/or soft tissue disease were excluded. DB population was adjusted for sex, MYCN amplification, disease type (relapsed, refractory), and disease site (bone marrow and/or bone) to balance aggregated characteristics of NAXI population. More characteristics were included in sensitivity analyses, including DB treatment without interleukin-2, as currently recommended. Overall response rate (ORR) was assessed as best response. Results: Aggregated data for NAXI from Study 201 (n = 52) and Study 230 (n = 38) and IPD from DB studies (APN311-202, APN311-304, c = 77) met the inclusion criteria. Compared to NAXI, DB significantly extended progression-free survival (PFS): hazard ratio, DB vs. NAXI of 0.47 (95% CI: 0.26 to 0.87, p = 0.015). ORR was 60.1% (95% CI: 48.5% to 71.6%) for DB vs. 43.3% (33.1% to 53.6%) for NAXI (ORR odds ratio, DB vs. NAXI was 1.97, 95% CI: 1.02 to 3.80, p = 0.044). Sensitivity analyses and unadjusted comparisons supported the results. Conclusion: In the indirect comparison, dinutuximab beta significantly extended PFS and increased ORR compared to naxitamab.
Keywords: dinutuximab beta; matching-adjusted indirect comparison; naxitamab; relapsed/refractory neuroblastoma; systematic review.
Conflict of interest statement
H.N.L., A.W., K.Ś., P.K., and P.H. have acted as consultants and participated in advisory boards organized by EUSA Pharma/Recordati Rare Diseases. J.G. has been a member of a DMC for a trial sponsored by YmAbs Therapeutics, and has had previous consulting/advisory board roles for EUSA Pharma, YmAbs Therapeutics, Celgene, Norgine and Abbvie. R.L. (Roberto Luksch) received travel reimbursement from Recordati Rare Diseases. All other authors do not declare any conflicts of interest.
Figures


References
-
- Matthay K.K., Villablanca J.G., Seeger R.C., Stram D.O., Harris R.E., Ramsay N.K., Swift P., Shimada H., Black C.T., Brodeur G.M., et al. Treatment of high-risk neuroblastoma with intensive chemotherapy, radiotherapy, autologous bone marrow transplantation, and 13-cis-retinoic acid. N. Engl. J. Med. 1999;341:1165–1173. doi: 10.1056/NEJM199910143411601. - DOI - PubMed
-
- Pritchard J., Cotterill S.J., Germond S.M., Imeson J., de Kraker J., Jones D.R. High dose melphalan in the treatment of advanced neuroblastoma: Results of a randomised trial (ENSG-1) by the European Neuroblastoma Study Group. Pediatr. Blood Cancer. 2005;44:348–357. doi: 10.1002/pbc.20219. - DOI - PubMed
-
- Abbas A.A., Samkari A.M.N. High-Risk Neuroblastoma: Poor Outcomes Despite Aggressive Multimodal Therapy. Curr. Cancer Ther. Rev. 2022;18:14–40. doi: 10.2174/1573394717666210805114226. - DOI
LinkOut - more resources
Full Text Sources
Miscellaneous

