Aromatase Inhibitors as Adjuvant Therapy in Early Breast Cancer: Insights into Toxicities and Their Management
- PMID: 40940824
- PMCID: PMC12427308
- DOI: 10.3390/cancers17172726
Aromatase Inhibitors as Adjuvant Therapy in Early Breast Cancer: Insights into Toxicities and Their Management
Abstract
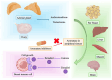
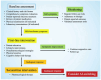
Aromatase inhibitors (AIs), with or without gonadotropin-releasing hormone analogs, are the cornerstone of adjuvant endocrine therapy for women with hormone receptor-positive early-stage breast cancer, offering significant reductions in recurrence risk and improving long-term survival. Their use is frequently accompanied by treatment-related toxicities that can adversely affect patients' quality of life (QoL) and adherence to therapy. Commonly reported side effects include vasomotor symptoms, such as hot flashes; musculoskeletal disorders, such as arthralgia and myalgia; mood disorders; and genitourinary discomfort, such as vaginal dryness and dyspareunia. Additionally, AIs are associated with a heightened risk of bone loss, leading to osteoporosis and fractures, and may have implications for cardiovascular health. Effective management of these adverse events is pivotal in maintaining treatment adherence and preserving QoL. Evidence-based strategies to address these toxicities include pharmacological interventions, such as analgesics for joint pain, bisphosphonates or denosumab for bone health, and hormonal or non-hormonal approaches for vasomotor and genitourinary symptoms. Non-pharmacological measures, including physical activity, dietary adjustments, and complementary therapies, can also help mitigate symptoms. This review examines the broad spectrum of AI-associated toxicities, discusses their clinical implications, and provides an overview of evidence-based management strategies. These insights aim to support clinicians in optimizing patient care while minimizing the toxicities of therapy.
Keywords: aromatase inhibitors; breast cancer survivorship; cardio-oncology; endocrine therapy toxicity; supportive cancer care.
Conflict of interest statement
No conflict of interest related to the present work.
Figures
References
-
- Allison K.H., Hammond M.E.H., Dowsett M., McKernin S.E., Carey L.A., Fitzgibbons P.L., Hayes D.F., Lakhani S.R., Chavez-MacGregor M., Perlmutter J., et al. Estrogen and Progesterone Receptor Testing in Breast Cancer: ASCO/CAP Guideline Update. J. Clin. Oncol. 2020;38:1346–1366. doi: 10.1200/JCO.19.02309. - DOI - PubMed
-
- Bradley R., Braybrooke J., Gray R., Hills R.K., Liu Z., Pan H., Peto R., Dodwell D., McGale P., Taylor C., et al. Aromatase Inhibitors versus Tamoxifen in Premenopausal Women with Oestrogen Receptor-Positive Early-Stage Breast Cancer Treated with Ovarian Suppression: A Patient-Level Meta-Analysis of 7030 Women from Four Randomised Trials. Lancet Oncol. 2022;23:382–392. doi: 10.1016/S1470-2045(21)00758-0. - DOI - PMC - PubMed
Publication types
LinkOut - more resources
Full Text Sources