Anti-EGFR Therapy in Metastatic Colorectal Cancer: Identifying, Tracking, and Overcoming Resistance
- PMID: 40940901
- PMCID: PMC12427569
- DOI: 10.3390/cancers17172804
Anti-EGFR Therapy in Metastatic Colorectal Cancer: Identifying, Tracking, and Overcoming Resistance
Abstract
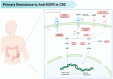
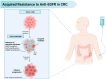
Epidermal growth factor receptor (EGFR) inhibitors remain a cornerstone in the treatment of metastatic colorectal cancer with RAS and BRAF wild-type cancer. Yet, primary and acquired resistance limit their benefit for many patients. A growing body of evidence reveals that resistance is not random but rather driven by a complex network of molecular alterations that sustain tumor growth independent of EGFR signaling. These include amplification of ERBB2 (HER2) and MET, activation of the PI3K and AKT pathways, EGFR extracellular domain mutations, and rare kinase fusions. The concept of negative hyperselection has emerged as a powerful strategy to refine patient selection by excluding tumors with these resistance drivers. Multiple clinical trials have consistently shown that patients who are hyperselected based on comprehensive molecular profiling achieve significantly higher response rates and improved survival compared to those selected by RAS and BRAF status alone. Liquid biopsy through circulating tumor DNA has further transformed this landscape, offering a noninvasive tool to capture tumor heterogeneity, monitor clonal evolution in real time, and guide rechallenge strategies after resistance emerges. Together, negative hyperselection, ctDNA-guided monitoring, and emerging therapeutics define a precision-oncology framework for identifying, tracking, and overcoming resistance to anti-EGFR therapy in mCRC, moving the field toward more effective and individualized care. Looking ahead, the development of innovative therapeutics such as bispecific antibodies, antibody drug conjugates, and RNA-based therapies promises to further expand in this challenging clinical scenario. These advances move precision oncology in colorectal cancer from concept to clinical reality, reshaping the standard of care through molecular insights.
Keywords: EGFR inhibition; circulating tumor DNA; metastatic colorectal cancer; negative hyperselection; precision oncology.
Conflict of interest statement
The authors declare no competing interests related to this work. All authors take responsibility for the accuracy and integrity of the data presented and their interpretation. Tiago Biachi de Castria receives an honorarium from Bristol Myers Squibb, Eli Lilly, Merck Sharp & Dohme Corp., AstraZeneca and A2Bio.
Figures

References
-
- GBD 2019 Colorectal Cancer Collaborators Global, regional, and national burden of colorectal cancer and its risk factors, 1990–2019: A systematic analysis for the Global Burden of Disease Study 2019. Lancet Gastroenterol. Hepatol. 2022;7:627–647. doi: 10.1016/S2468-1253(22)00044-9. - DOI - PMC - PubMed
-
- Sung H., Siegel R.L., Laversanne M., Jiang C., Morgan E., Zahwe M., Cao Y., Bray F., Jemal A. Colorectal cancer incidence trends in younger versus older adults: An analysis of population-based cancer registry data. Lancet Oncol. 2025;26:51–63. doi: 10.1016/S1470-2045(24)00600-4. - DOI - PMC - PubMed
-
- Guo L., Wang Y., Yang W., Wang C., Guo T., Yang J., Shao Z., Cai G., Cai S., Zhang L., et al. Molecular Profiling Provides Clinical Insights into Targeted and Immunotherapies as Well as Colorectal Cancer Prognosis. Gastroenterology. 2023;165:414–428.E7. doi: 10.1053/j.gastro.2023.04.029. - DOI - PubMed
Publication types
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

