Clinical Utility of the EpiSwitch CiRT Test to Guide Immunotherapy Across Solid Tumors: Interim Results from the PROWES Study
- PMID: 40940997
- PMCID: PMC12427751
- DOI: 10.3390/cancers17172900
Clinical Utility of the EpiSwitch CiRT Test to Guide Immunotherapy Across Solid Tumors: Interim Results from the PROWES Study
Abstract
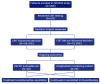
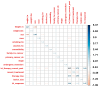
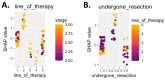
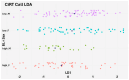
Background: Immunotherapy has revolutionized oncology care, but clinical response to immune checkpoint inhibitors (ICIs) remains unpredictable, and treatment carries substantial risks and costs. The EpiSwitch® CiRT blood test is a novel 3D genomic assay that stratifies patients by probability of ICI benefit using a binary, blood-based classification: high (HPRR) or low (LPRR) probability of response. Methods: This interim analysis of the ongoing PROWES prospective real-world evidence study evaluates the clinical utility of CiRT in 205 patients with advanced solid tumors. The primary endpoint was treatment decision impact, assessed by pre-/post-test physician surveys. Secondary endpoints included treatment avoidance, time to ICI initiation, concordance with clinical response, early discontinuation rates, and exploratory health economic modeling. Longitudinal use, resistance monitoring, and equity analysis by social determinants of health (SDoH) were also explored. Results: CiRT results influenced clinical decision-making in a majority of cases. LPRR status was associated with higher rates of treatment avoidance and early discontinuation due to immune-related adverse events (IrAEs). In contrast, HPRR patients experienced greater clinical benefit and longer ICI exposure. CiRT classification was not associated with short-term imaging-based response outcomes, supporting its role as an independent predictor. Given that ICI therapy and supportive care can cost more than $850,000 per patient, CiRT offers potential value in avoiding ineffective treatment and associated toxicities. Conclusions: CiRT demonstrates meaningful clinical utility as a non-invasive, predictive tool for guiding immunotherapy decisions across tumor types. It enables more precise treatment selection, improves patient outcomes, and supports value-based cancer care.
Keywords: 3D genome conformation; EpiSwitch CiRT assay; blood-based cancer diagnostics; checkpoint inhibitors; immunotherapy response; precision oncology; predicting checkpoint inhibitor response; real-world evidence.
Conflict of interest statement
Joe Abdo and Thomas Guiel are full-time paid employees of Oxford BioDynamics, Inc. in the USA. Robert Heaton is a part-time paid Medical Director of Oxford BioDynamics, Inc. in the USA. Ewan Hunter and Alexandre Akoulitchev are full-time paid employees of Oxford BioDynamics, PLC in the UK. The authors have no other relevant affiliations or financial involvement with any organization or entity with financial interest or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed. No writing assistance was utilized in the production of this manuscript.
Figures

References
-
- Li J.X., Huang J.M., Jiang Z.B., Li C.Q., Wang X.L., Chen M., Xu J., Li J., Zhou Q., Wang Z., et al. Current clinical progress of PD-1/PD-L1 immunotherapy and potential combination treatment in non-small cell lung cancer. Integr. Cancer Ther. 2019;18:1–13. doi: 10.1177/1534735419890020. - DOI - PMC - PubMed
LinkOut - more resources
Full Text Sources

