Palatal Abscess of Endodontic Origin with Extensive Radiolucency in Maxillary CBCT Imaging
- PMID: 40941683
- PMCID: PMC12428685
- DOI: 10.3390/diagnostics15172195
Palatal Abscess of Endodontic Origin with Extensive Radiolucency in Maxillary CBCT Imaging
Abstract
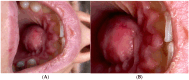
Palatal abscesses of endodontic origin are rarer than buccal ones due to maxillary anatomy. Their clinical appearance may resemble that of other palatal illnesses, complicating diagnosis and treatment. Prevention of problems requires early detection and endodontic treatment. A 26-year-old female patient presented with a 2 cm diameter palatal abscess, significant pulsatile discomfort, fever, and enlargement of the anterior hard palate. Clinical examination showed grade 1 mobility of the central and lateral incisors, percussion discomfort, and negative pulp vitality in the case of the lateral incisor. Cone-beam computed tomography (CBCT) showed two radiolucent lesions: a posterior cystic lesion near the first molar and an anterior lesion near the upper left lateral incisor. Palatal cortical bone puncture and soft tissue extension indicated the abscess origin. According to the clinical and imaging evaluation, the upper left lateral incisor had a persistent periapical lesion of endodontic origin that a palatal abscess with cortical bone perforation had exacerbated.
Keywords: CBCT; cortical bone perforation; endodontic infection; palatal abscess.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures


References
-
- Pellecchia R., Holmes C., Barzani G., Sebastiani F.R. Antimicrobial Resistance—A Global Threat. InTech; London, UK: 2016. Antimicrobial Therapy and Surgical Management of Odontogenic Infections. - DOI
LinkOut - more resources
Full Text Sources

