Micropropagation of Apple Cultivars 'Golden Delicious' and 'Royal Gala' in Bioreactors
- PMID: 40941902
- PMCID: PMC12430669
- DOI: 10.3390/plants14172740
Micropropagation of Apple Cultivars 'Golden Delicious' and 'Royal Gala' in Bioreactors
Abstract
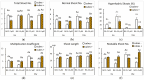
This study aimed to investigate culture conditions for the efficient micropropagation of apple cultivars 'Golden Delicious' and 'Royal Gala' in liquid medium by temporary immersion. RITA® bioreactors were used for the multiplication stage whereas RITA® or Plantform™ were used for the rooting stage. Murashige and Skoog media (MS) with N6-benzyladenine (BA) was used for shoot multiplication and indole-3-butyric acid (IBA) for root induction. During the multiplication phase, we evaluated the mineral medium, BA concentration, immersion frequency, silver nitrate and activated charcoal supplementation and the use of physical supports to hold explants in an upright position. The results demonstrated that longer incubation periods (10 weeks) were better than shorter periods (6 weeks) for decreasing hyperhydricity and increasing the multiplication coefficient (MC). For 'Golden Delicious', the highest MC were obtained either with explants placed directly on the bioreactor basket and immersed six times per day for 60 s in MS with 2.2 µM BA or explants placed between rockwool cubes cultivated with 4.4 µM BA (both yielding MC of 8.9 and 5-10% hyperhydricity). These results were superior to 'Royal Gala', which showed a MC of 7.3 and 23% of hyperhydricity when cultivated in MS with half nitrates, 1.55 µM BA and rockwool cubes. Both varieties rooted efficiently (96-100%), and resulting plantlets were successfully acclimated. This is the first report in the micropropagation of these two commercial fruiting cultivars in temporary immersion, demonstrating the potential of this technology to enhance large-scale plant production for the apple nursery industry.
Keywords: Malus domestica; hyperhydricity; in vitro; multiplication; rooting; temporary immersion.
Conflict of interest statement
The author Bruce Christie was employed by the company “The Greenplant Company”. The remaining authors declare that the research was conducted in the absence of any commercial or financial relationship that could be construed as a potential conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript; or in the decision to publish the results.
Figures








References
-
- Fruit Production by Variety Worldwide 2023. [(accessed on 28 July 2025)]. Available online: https://www.statista.com/statistics/264001/worldwide-production-of-fruit...
-
- FAOSTAT [(accessed on 28 July 2025)]. Available online: https://www.fao.org/faostat/en/#data/QV/visualize.
-
- OECD . Unclassified Environment Directorate Joint Meeting of the Chemicals Committee and the Working Party on Chemicals, Pesticides and Biotechnology. Consensus Document on the Biology of Apple (Malus domestica Borkh.) Volume 66. OECD; Paris, France: 2019. [(accessed on 11 August 2025)]. pp. 1–51. (Series on Harmonisation of Regulatory Oversight in Biotechnology). Available online: https://one.oecd.org/document/ENV/JM/MONO(2019)30/En/pdf.
Grants and funding
LinkOut - more resources
Full Text Sources

