COSMO-RS Solubility Screening and Coumarin Extraction from Pterocaulon polystachyum with Deep Eutectic Solvents
- PMID: 40941996
- PMCID: PMC12429901
- DOI: 10.3390/molecules30173468
COSMO-RS Solubility Screening and Coumarin Extraction from Pterocaulon polystachyum with Deep Eutectic Solvents
Abstract
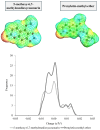
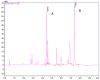
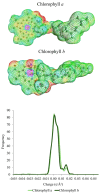
Deep eutectic solvents (DESs) have been studied to obtain extracts from medicinal plants, aiming for a more environmentally friendly process. Aligned with this initiative, the use of predictive thermodynamic models for screening the best solvent represents a theoretical action to reduce experimental time and cost. Therefore, this study aimed to perform and validate a relative solubility screening of 5-methoxy-6,7-methylenedioxycoumarin and prenyletin-methyl-ether at 313 K in choline chloride, menthol, and betaine-based DES, using the COSMO-RS model in COSMOThermX software. The density of DES was also predicted with a maximum error of 7.31% for this property. Ultrasound-assisted extraction (UAE) with DES at 313 K, 30 min, and a solid/liquid ratio of 1:20 (w/w) was performed to confirm the theoretical solubility results experimentally, as the extracts were analyzed through ultrafast liquid chromatography (UFLC) for coumarin content. For the results, the coumarin molecules presented intense peaks in the nonpolar region of their σ-profile, and the relative solubility screening indicated the DES Men/Lau (2:1), known for its hydrophobic nature and low polarity, as the best DES to solubilize these coumarins. Nevertheless, the UFLC results, and the complementary solubility screening of pigments, showed an interaction preference of this DES with chlorophylls instead of coumarins. This result was corroborated by spectrophotometric analysis of the extracts in UV-Vis, demonstrating that experimental validation is still mandatory in extraction processes and that predictive methodologies such as COSMO-RS should be used as guiding tools and analyzed in a greater context, considering the complexity of plant matrices in the beginning of simulations.
Keywords: coumarin; emerging solvents; predictive model; relative solubility.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures



References
-
- Abdussalam-Mohammed W., Ali A.Q., Errayes A.O. Green Chemistry: Principles, Applications, and Disadvantages. Chem. Methodol. 2020;4:408–423. doi: 10.33945/sami/chemm.2020.4.4. - DOI
-
- Benvenutti L., Zielinski A.A.F., Ferreira S.R.S. Which Is the Best Food Emerging Solvent: IL, DES or NADES? Trends Food Sci. Technol. 2019;90:133–146. doi: 10.1016/j.tifs.2019.06.003. - DOI
-
- Martins M.A.R., Pinho S.P., Coutinho J.A.P. Insights into the Nature of Eutectic and Deep Eutectic Mixtures. J. Solution Chem. 2019;48:962–982. doi: 10.1007/s10953-018-0793-1. - DOI
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

