Lattice Structures in Additive Manufacturing for Biomedical Applications: A Systematic Review
- PMID: 40942202
- PMCID: PMC12430481
- DOI: 10.3390/polym17172285
Lattice Structures in Additive Manufacturing for Biomedical Applications: A Systematic Review
Abstract
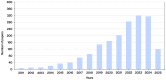
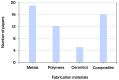
The present study offers a systematic review of the current state of research on lattice structures manufactured by additive technologies for biomedical applications, with the aim of identifying common patterns, such as the use of triply periodic minimal surfaces (TPMS) for bone scaffolds, as well as technological gaps and future research opportunities. Employing the PRISMA (Preferred Reporting Items for Systematic Reviews and Meta-Analyses) methodology, the review process ensures methodological rigor and replicability across the identification, screening, eligibility, and inclusion phases. Additionally, PRISMA was tailored by prioritizing technical databases and engineering-specific inclusion criteria, thereby aligning the methodology with the scope of this field. In recent years, a substantial surge in interdisciplinary research has underscored the promise of architected porous structures in enhancing mechanical compatibility, fostering osseointegration, and facilitating personalized medicine. A growing body of literature has emerged that explores the optimization of geometric features to replicate the behavior of biological tissues, particularly bone. Additive manufacturing (AM) has played a pivotal role in enabling the fabrication of complex geometries that are otherwise unachievable by conventional methods. The applications of lattice structures range from permanent load-bearing implants, commonly manufactured through selective laser melting (SLM), to temporary scaffolds for tissue regeneration, often produced with extrusion-based processes such as fused filament fabrication (FFF) or direct ink writing (DIW). Notwithstanding these advances, challenges persist in areas such as long-term in vivo validation, standardization of mechanical and biological testing, such as ISO standards for fatigue testing, and integration into clinical workflows.
Keywords: 3D printing; additive manufacturing; architected cellular materials; biomaterials; biomedical applications; lattice structures; systematic review.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures













References
-
- Nazir A., Abate K.M., Kumar A., Jeng J.Y. A state-of-the-art review on types, design, optimization, and additive manufacturing of cellular structures. Int. J. Adv. Manuf. Technol. 2019;104:3489–3510. doi: 10.1007/s00170-019-04085-3. - DOI
-
- Borikar G.P., Patil A.R., Kolekar S.B. Additively Manufactured Lattice Structures and Materials: Present Progress and Future Scope. Int. J. Precis. Eng. Manuf. 2023;24:2133–2180. doi: 10.1007/s12541-023-00848-x. - DOI
-
- Pan C., Han Y., Lu J. Design and Optimization of Lattice Structures: A Review. Appl. Sci. 2020;10:6374. doi: 10.3390/app10186374. - DOI
-
- Ahmad A., Belluomo L., Bici M., Campana F. Bird’s Eye View on Lattice Structures: Design Issues and Applications for Best Practices in Mechanical Design. Metals. 2023;13:1666. doi: 10.3390/met13101666. - DOI
Publication types
LinkOut - more resources
Full Text Sources

