Curve-Based Infill Pattern Optimization for 3D Printed Polymeric Scaffolds for Trabecular Bone Applications
- PMID: 40942481
- PMCID: PMC12429743
- DOI: 10.3390/ma18174055
Curve-Based Infill Pattern Optimization for 3D Printed Polymeric Scaffolds for Trabecular Bone Applications
Abstract
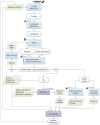
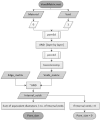
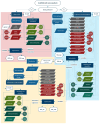
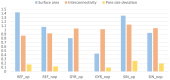
Additive manufacturing technology, specifically material extrusion, offers great potential for scaffold manufacturing in tissue engineering. This study presents a novel methodology for the design and optimization of 3D printed polymeric scaffolds to enhance cell viability, thereby promoting improved cell proliferation for tissue engineering applications. Different infill patterns, including gyroid, parallel sinusoidal, and symmetric sinusoidal, were evaluated to determine their impact on cell proliferation and tissue regeneration. To overcome the limitations of existing slicer software, a novel open-source software called FullControl GCode Designer was utilized, enabling the creation of customized infill patterns without restrictions. VOLCO software was employed to generate voxelized 3D models of the scaffolds, simulating the material extrusion process. Finite element analysis was conducted using Abaqus software to evaluate the mechanical properties of the different designs. Additionally, new scripts were developed to evaluate the interconnectivity and pore size of the voxelized models. A factorial design of experiments and a genetic algorithm (combined with Kriging metamodels) were applied to identify the optimal configuration based on optimization criteria (keeping the mechanical stiffness and pore size within the recommended values for trabecular bone and maximizing the surface and interconnectivity). Biological testing was conducted on polylactic acid scaffolds to preliminarily validate the effectiveness of the modeling and optimization methodologies in this regard. The results demonstrated the agreement between the optimization methodology and the biological test since the optimum in both cases was a symmetric sinusoidal pattern design with a configuration resulting in a structure with 53.08% porosity and an equivalent pore size of 584 µm. Therefore, this outcome validates the proposed methodologies, emphasizing the role of pore surface area and interconnectivity in supporting cell proliferation. Overall, this research contributes to the advancement of AM technology in tissue engineering and paves the way for further optimization studies in scaffold design.
Keywords: FEA; additive manufacturing; modeling; optimization; scaffold; tissue engineering.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures










References
-
- Naghieh S., Karamooz Ravari M.R., Badrossamay M., Foroozmehr E., Kadkhodaei M., Karamooz Ravari M.R., Badrossamay M., Foroozmehr E., Kadkhodaei M. Numerical Investigation of the Mechanical Properties of the Additive Manufactured Bone Scaffolds Fabricated by FDM: The Effect of Layer Penetration and Post-Heating. J. Mech. Behav. Biomed. Mater. 2016;59:241–250. doi: 10.1016/j.jmbbm.2016.01.031. - DOI - PubMed
-
- Qu H. Additive Manufacturing for Bone Tissue Engineering Scaffolds. Mater. Today Commun. 2020;24:101024. doi: 10.1016/j.mtcomm.2020.101024. - DOI
-
- Singh D., Babbar A., Jain V., Gupta D., Saxena S., Dwibedi V. Synthesis, Characterization, and Bioactivity Investigation of Biomimetic Biodegradable PLA Scaffold Fabricated by Fused Filament Fabrication Process. J. Braz. Soc. Mech. Sci. Eng. 2019;41:121. doi: 10.1007/s40430-019-1625-y. - DOI
Grants and funding
LinkOut - more resources
Full Text Sources

