Rodent Models of Lung Disease: A Road Map for Translational Research
- PMID: 40943307
- PMCID: PMC12429320
- DOI: 10.3390/ijms26178386
Rodent Models of Lung Disease: A Road Map for Translational Research
Abstract
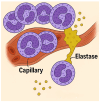
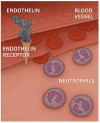
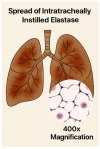
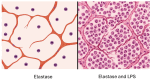
Animal models provide a controlled and reproducible environment for investigating the pathogenesis of human lung diseases. In many cases, the morphological changes associated with a particular model may resemble those seen in their human counterparts, but the corresponding biochemical events may differ, and their timeframe may be significantly reduced. Nevertheless, gaining insight into human disease mechanisms may be possible by employing experimental approaches that minimize the problems associated with extrapolating data from animal studies. Such strategies include using more than one model of a particular disease, employing different routes of administration of the injurious agent, using a variety of animal strains or species, or focusing on biochemical mechanisms common to both the animal model and its human counterpart. For example, rodent models that replicate elastic fiber injury in human pulmonary emphysema have been used to test aerosolized hyaluronan's ability to slow the disease's progression. The same models facilitated the identification of a new biomarker for pulmonary emphysema that may be a real-time indicator of therapeutic efficacy in clinical trials. Therefore, the appropriate use of these models can provide a necessary road map for designing appropriate dosages, delivery routes, timeframes, and endpoints in clinical trials of novel agents for the treatment of lung disease.
Keywords: ALI; animal models; hyaluronan; pulmonary emphysema; pulmonary fibrosis.
Conflict of interest statement
The authors declare no conflict of interest.
Figures





References
-
- Tanner L., Single A.B. Animal models reflecting chronic obstructive pulmonary disease and related respiratory disorders: Translating pre-clinical data into clinical relevance. [(accessed on 24 May 2025)];J. Innate Immun. 2020 12:203–225. doi: 10.1159/000502489. Available online: https://karger.com/jin/article/12/3/203/180041. - DOI - PMC - PubMed
-
- Jenkins R.G., Moore B.B., Chambers R.C., Eickelberg O., Fernandez I.E., Hogaboam C.M., Kolls J.K., Kolb M., Lawson W.E., Lloyd C.M., et al. An official American Thoracic Society workshop report: Use of animal models for the preclinical assessment of potential therapies for pulmonary fibrosis. Am. J. Respir. Cell Mol. Biol. 2017;57:P1–P10. doi: 10.1165/rcmb.2017-0096ST. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

