Lignin-Derived Oligomers as Promising mTOR Inhibitors: Insights from Dynamics Simulations
- PMID: 40943647
- PMCID: PMC12429072
- DOI: 10.3390/ijms26178728
Lignin-Derived Oligomers as Promising mTOR Inhibitors: Insights from Dynamics Simulations
Abstract
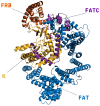
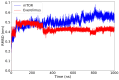
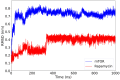
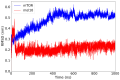
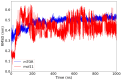
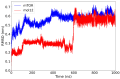
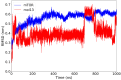
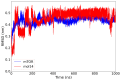
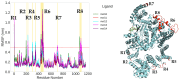
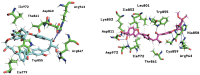
The mammalian target of rapamycin pathway, mTOR, is a crucial signaling pathway that regulates cell growth, proliferation, metabolism, and survival. Due to its dysregulation it is involved in several ailments such as cancer or age-related diseases. The discovery of mTOR and the understanding of its biological functions were greatly facilitated by the use of rapamycin, an antibiotic of natural origin, which allosterically inhibits mTORC1, effectively blocking its function. In this entirely computational study, we investigated mTOR's interaction with seven ligands: two clinically established inhibitors (everolimus and rapamycin) and five lignin-derived oligomers, a renewable natural polyphenol recently used for the drug delivery of everolimus. The seven complexes were analyzed through all-atom molecular dynamics simulations in explicit solvent using a high-performance computing platform. Trajectory analyses revealed stable interactions between mTOR and all ligands, with lignin-derived compounds showing comparable or enhanced binding stability relative to reference drugs. To evaluate the stability of the molecular complex and the behavior of the ligand over time, we analyzed key parameters including root mean square deviation, root mean square fluctuation, number of hydrogen bonds, binding free energy, and conformational dynamics assessed through principal component analysis. Our results suggest that lignin fragments are a promising, sustainable scaffold for developing novel mTOR inhibitors.
Keywords: MM/PBSA binding free energy; everolimus; high-performance computing (HPC); hydrophobic and hydrogen bond interactions; lignin-derived oligomers; mTOR; mTOR inhibitors; molecular dynamics (MD) simulations; rapamycin.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures



References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous

