Dual targeting of solid tumors using cytokine-induced killer cells modified with a CAR anti-tenascin C and a secretable EGFRxCD3 bispecific antibody
- PMID: 40944718
- PMCID: PMC12433391
- DOI: 10.1007/s00262-025-04149-2
Dual targeting of solid tumors using cytokine-induced killer cells modified with a CAR anti-tenascin C and a secretable EGFRxCD3 bispecific antibody
Abstract
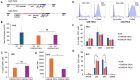
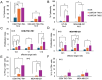
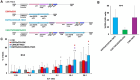
To generate CARCIK cells with enhanced targeting and penetration of solid tumors, we have designed new CAR molecules against tenascin C (TNC), an extracellular matrix and surface molecule, overexpressed in several tumor types. Two different anti-TNC CAR constructs were generated, sharing the anti-TNC scFv domain, fused to the CD3ζ fragment, but differing in co-signaling domains: CAR-TNC4 carrying the 4-1BB and CAR-TNC5 the CD28 and OX40 domains. Both CARs were introduced into cytokine-induced killer cells (CIK) by co-transfection with the sleeping beauty transposase plasmid. CARCIK-TNC cells were cytotoxic against TNC+ targets, proliferated and secreted the IFN-γ and IL-2 cytokines in response to target cell binding, with overall higher efficacy of CARCIK-TNC5 compared to CARCIK-TNC4. To enhance activity and specificity, we also generated a dual construct, carrying a secretable EGFRxCD3 bispecific T cell engager (sBiTE) antibody cDNA. CIK transfected with EGFRxCD3/CAR-TNC5 showed good expression of both molecules and synergistic killing of MDA-MB-231 cells in vitro compared to cells transfected with the single-targeting molecules. Also in vivo, in the subcutaneous TNC+ EGFR+ MDA-MB-231 xenograft model, significantly enhanced control of tumor growth was observed after injection of CIK cells transfected with dual, compared to single constructs. We conclude that CAR-TNC5 combined with EGFRxCD3 can endow CIK cells with different and potentially synergistic functions in vivo.
Keywords: Bispecific T cell engager; Chimeric antigen receptor; Cytokine induced killer; Tenascin C; solid tumor.
© 2025. The Author(s).
Conflict of interest statement
Declarations. Conflict of interests: AR has received honoraria for lectures or presentations from Novartis, Amgen, Pfizer, Astellas, Jazz, Janssen, Incyte, Kite-Gilead, Omeros, Sanofi, Italfarmaco; has received support for attending meetings and/or travel from Novartis, Amgen, Pfizer, Astellas, Jazz, Janssen, Incyte, Kite-Gilead, Omeros; participated on a Data Safety Monitoring Board or Advisory Board from Novartis, Amgen, Pfizer, Astellas, Jazz, Janssen, Incyte, Kite-Gilead, Roche, Omeros, Sanofi, Italfarmaco.
Figures


References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

