Dynamics of RSV hospitalization rates in ≤ 2-year-old children between 2020-2023 in Europe
- PMID: 40944779
- PMCID: PMC12433331
- DOI: 10.1007/s00431-025-06218-1
Dynamics of RSV hospitalization rates in ≤ 2-year-old children between 2020-2023 in Europe
Abstract
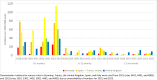
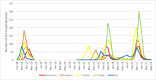
With the newly approved RSV preventive strategies enabling universal protection of infants, it is crucial to gain a comprehensive understanding of RSV hospitalization incidence, prior to the introduction of these strategies in order to facilitate an assessment of their impact. Children ≤ 2 years hospitalized with laboratory-confirmed RSV infection between 2020-2023 in France, Germany, Spain, Italy, and United Kingdom were included and compared with the 2018-2019 season. The population-based incidence was calculated as number of RSV hospitalizations divided by market share-adjusted number of children in the catchment area. Across participating countries, we observed a decrease in RSV hospitalization incidence during the 2020-2021 season due to the COVID-19 pandemic, dropping to 5.9/1000 child-years (95%CI 5.4-6.3) compared with 11.3/1000 child-years (95%CI 10.6-11.9) in 2018-2019. This decline was followed by a rebound in incidence, with rates reaching 13.8/1000 child-years (95%CI 13.0-14.5) in 2021-2022 and 18.8/1000 child-years (95%CI 18.0-19.7) in 2022-2023. Distinct patterns of RSV resurgence were observed across countries. During the 2020-2021 season, there was an increase in PICU admissions (29.5% vs 20.0% pre-pandemic, p < 0.001), despite a lower total number of RSV admissions (610 vs 1,238) compared to the 2018-2019 season.
Conclusions: The population-based incidence of RSV hospitalization in children ≤ 2 years is substantial. Considerable variation in incidence was observed between 2020 and 2023, with an initial decline during the COVID-19 pandemic followed by a rebound in the subsequent seasons. Our study underscores the importance of RSV surveillance and flexibility in RSV preventive strategies.
What is known: • RSV is a major cause of hospitalization in young children under 5 years of age worldwide. • RSV seasonality was disrupted during the COVID-19 pandemic.
What is new: • Distinct patterns of RSV resurgence were observed across five European countries during the COVID-19 pandemic, with an initial decline in incidence of RSV associated hospitalizations in children ≤ 2 years, followed by a rebound in the subsequent seasons, reaching 18.8 per 1,000 child-years (95% CI: 18.0 - 19.7) in 2022-2023.
Keywords: COVID-19 pandemic; Children; Population-based incidence; RSV; RSV hospitalization.
© 2025. Merck & Co., Inc., Rahway, NJ, USA and its affiliates, and the Authors 2025.
Conflict of interest statement
Declarations. Competing interest: Yoonyoung Choi and Madelyn Ruggieri are employees at Merck Sharp & Dohme LLC, a subsidiary of Merck & Co., Inc., Rahway, NJ, USA. FM-T has acted as principal investigator in randomized controlled trials of Ablynx, Abbot, Seqirus, Sanofi Pasteur, MSD, Pfizer, Roche, Regeneron, Jansen, Medimmune, Novavax, Novartis and GSK, with honoraria paid to his institution. FM-T reports a relationship with GSK Vaccines SRL that includes consulting or advisory. FM-T reports a relationship with Pfizer Inc that includes consulting or advisory. FM-T reports a relationship with Sanofi Pasteur Inc that includes consulting or advisory. FM-T reports a relationship with Janssen Pharmaceuticals Inc that includes consulting or advisory. FM-T reports a relationship with MSD that includes consulting or advisory. FM-T reports a relationship with Seqirus Pty Ltd that includes consulting or advisory. SBD has previously received honoraria from Sanofi for taking part in RSV advisory boards and has provided consultancy and/or investigator roles in relation to product development for Janssen, AstraZeneca, Pfizer, Moderna, Valneva, MSD, iLiAD and Sanofi with fees paid to my institution. SBD is a member of the UK Department of Health and Social Care’s (DHSC) Joint Committee on Vaccination and Immunisation (JCVI) RSV subcommittee and Medicines and Healthcare products Regulatory Agency’s (MHRA) Paediatric Medicine Expert Advisory Group (PMEAG), but the reviews expressed herein do not necessarily represent those of DHSC, JCVI, MHRA or PMEAG. JGW has been an investigator for clinical trials sponsored by pharmaceutical companies including AstraZeneca, MSD, Pfizer, Sanofi, and Janssen and has been an investigator for clinical trials funded by IMI/Horizon2020 and ZonMw. All funds have been paid to UMCU. JGW participated in advisory boards of Janssen and Sanofi and was a speaker at Sanofi and MSD sponsored symposia with honoraria paid to UMCU. LB has regular interaction with pharmaceutical and other industrial partners. He has not received personal fees or other personal benefits. UMCU has received major funding (> €100,000 per industrial partner) for investigator initiated studies from AstraZeneca, Sanofi, Janssen, Pfizer, MSD and MeMed Diagnostics. UMCU has received major funding from the Bill and Melinda Gates Foundation. UMCU has received major funding as part of the public private partnership IMI-funded RESCEU and PROMISE projects with partners GSK, Novavax, Janssen, AstraZeneca, Pfizer and Sanofi. UMCU has received major funding by Julius Clinical for participating in clinical studies sponsored by AstraZeneca, Merck and Pfizer. UMCU received minor funding (€1000–25,000 per industrial partner) for consultation, DSMB membership or invited lectures by Ablynx, Bavaria Nordic, GSK, Novavax, Pfizer, Moderna, Astrazeneca, MSD, Sanofi, Janssen. LB is the founding chairman of the ReSViNET Foundation. EH has received speaking fees and travel support from Pfizer, MSD, Sanofi, Abbvie/AstraZeneca and Chiesi. RE has previously received honoraria for lectures, expertise or educational events. RE has previously received honoraria from AstraZeneca and Sanofi for taking part in RSV advisory boards and also from AstraZeneca, GSK Pfizer, and Sanofi for lectures, expertise or educational events. JL has been an investigator for clinical trials sponsored by pharmaceutical companies including AstraZeneca, GSK, MSD, Pfizer and Sanofi and received consulting or lecture fees from Enanta, Engelhard Pharma, Gilead Sciences, MSD, Pfizer, Sanofi and Siemens Health Care. CA has received fees for meetings or consulting or advisory boards from Astra Zeneca, GSK, MSD, Moderna, Novavax, Pfizer, Sanofi.
Figures

References
-
- European Medicines Agency. News. New medicine to protect babies and infants from respiratory syncytial virus (RSV) infection. Sept 16, 2022. https://www.ema.europa.eu/en/news/new-medicine-protect-babies-infants-re... (Accessed Sept 9, 2023)
MeSH terms
LinkOut - more resources
Full Text Sources
Medical

